Abstract
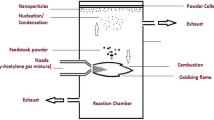
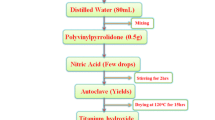
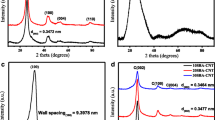
Titanium dioxide (TiO2) and mixed oxides, i.e., mixtures of magnesium oxide and titanium dioxide (MgO–TiO2) with different ratios were synthesized by two methods—flame synthesis and aerogel, for comparison of their properties. The samples were characterized by powder x-ray diffraction (pXRD), energy-dispersive x-ray spectroscopy, Fourier transform infrared spectroscopy, Brunauer-Emmet-Teller method of surface area measurements, ultraviolet-visible spectroscopy (UV-vis), and transition electron microscopic analysis. The pXRD patterns of different mixed oxides with different mole ratios revealed that there were formations of different compositions and phases. These mixed oxides were also used as photocatalysts in the UV-vis light to oxidize acetaldehyde, and carbon dioxide (CO2) was measured as a product. The mixed oxides with low content of MgO (∼1–2 mol%) were found to be more UV-active photocatalysts for the degradation of acetaldehyde than the degradation by Degussa P25 and as-synthesized TiO2, the highest by the MgO–TiO2 mixed oxides of 1:50 ratio when comparisons were carried out among the samples prepared by the same method. Furthermore, the mixed oxides prepared by the aerogel method were found to be superior photocatalysts compared with the mixed oxides of equal ratio prepared by flame synthesis. This effect of insulator, MgO, on the photocatalytic activity of semiconductor, TiO2, was found to be interesting and can be applied for other applications as environmentally friendly materials.






Similar content being viewed by others
References
A.E. Jacobsen: Titanium dioxide pigments. Ind. Eng. Chem. 41, 523 (1949).
E. Pramauro, M. Vincenti, V. Augugliaro, and L. Palmisano: Photocatalytic degradation of monuron in aqueous TiO2 dispersions. Environ. Sci. Technol. 27, 1790 (1993).
Y. Zhang, J.C. Crittenden, D.W. Hand, and D.L. Perram: Fixed-bed photocatalysts for solar decontamination of water. Environ. Sci. Technol. 28, 435 (1994).
J. Liu, Y. Hu, F. Gu, and C. Li: Flame synthesis of ball-in-shell structured TiO2 nanospheres. Ind. Eng. Chem. Res. 48, 735 (2009).
K.T. Lim, H.S. Hwang, W. Ryoo, and K.P. Johnston: Synthesis of TiO2 nanoparticles utilizing hydrated reverse micelles in CO2. Langmuir 20, 2466 (2004).
S.M. Liu, L.M. Gan, L.H. Liu, W.D. Zhang, and H.C. Zeng: Synthesis of single-crystalline TiO2 nanotubes. Chem. Mater. 14, 1391 (2002).
M. Zhang, Y. Bando, and K. Wada: Sol-gel template preparation of TiO2 nanotubes and nanorods. J. Mater. Sci. Lett. 20, 167 (2001).
C.A. Grimes: Synthesis and application of highly ordered arrays of TiO2 nanotubes. J. Mater. Chem. 17, 1451 (2007).
H. Yin, Y. Wada, T. Kitamura, S. Kambe, S. Murasawa, H. Mori, T. Sakata, and S. Yanagida: Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 11, 1694 (2001).
A.V. Murugan, V. Samuel, and V. Ravi: Synthesis of nanocrystalline anatase TiO2 by microwave hydrothermal method. Mater. Lett. 60, 479 (2006).
A. Goossens, E.L. Maloney, and J. Schoonman: Gas-phase synthesis of nanostructured anatase TiO2. Chem. Vap. Deposition 4, 109 (1998).
T. Ohno, K. Takieda, S. Higashida, and M. Matsumura: Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal., A 244, 383 (2003).
T. Kawahara, Y. Konishi, H. Tada, N. Tohge, J. Nishii, and S. Ito: A patterned TiO2 (anatase)/TiO2 (rutile) bilayer-type photocatalyst: Effect of the anatase/rutile junction on the photocatalytic activity. Angew. Chem. Int. Ed. 114, 2935 (2002).
T. Ohno, K. Surukawa, and M. Matsumura: Photocatalytic activities of pure rutile particles isolated from TiO2 powder by dissolving the anatase component in HF solution. J. Phys. Chem. B 105, 2417 (2001).
Q. Sun and Y. Xu: Evaluating intrinsic photocatalytic activities of anatase and rutile TiO2 for organic degradation in water. J. Phys. Chem. C 114, 18911(2010).
T. Ohno, K. Sarukawa, and M. Matsumura: Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reaction. New J. Chem. 26, 1167 (2002).
O.K. Varghese, M. Paulose, T.J. LaTempa, and C.A. Grimes: High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 9, 731 (2009).
D.B. Hamal and K.J. Klabunde: Synthesis, characterization, and visible light activity of new nanoparticle photocatalysts based on silver, carbon, and sulfur-doped TiO2. J. Colloid Interface Sci. 311, 514 (2007).
A.D. Paola, E.G. Lopez, S. Ikeda, G. Marci, B. Ohtani, and L. Palmisano: Photocatalytic degradation of organic compounds in aqueous systems by transition metal-doped polycrystalline TiO2. Catal. Today 75, 87 (2002).
J.C. Bailor, H.J. Emeléus, R. Nyholm, and A.F. Trotman-Dikenson: Comprehensive Inorganic Chemistry, 1st ed. (Compendium Publishers, Elmsford, NY, 1, 1973).
J. Zhan, Y. Bando, J. Hu, and D. Golberg: Bulk synthesis of single-crystalline magnesium oxide nanotubes. Inorg. Chem. 43, 2462 (2004).
R. Richards, R.S. Mulukutla, I. Mishakov, V. Chesnokov, A. Volodin, V. Zaikovski, N. Sun, and K.J. Klabunde: Nanocrystalline ultrahigh surface area magnesium oxide as a selective base catalyst. Scr. Mater. 44, 1663–1666 (2001).
R.M. Narske, K.J. Klabunde, and S. Fultz: Solvent effect on the heterogeneous adsorption and reaction of (2-chloroethyl )ethyl sulfide on nanocrystalline magnesium oxide. Langmuir 18, 4819 (2002).
R. Richards, W. Li, S. Decker, C. Davidson, O. Koper, V. Zaikovski, A. Volodin, T. Rieker, and K.J. Klabunde: Consolidation of metal oxide nanocrystals. Reactive pellets with controllable pore structure that represent a new family of porous, inorganic materials. J. Am. Chem. Soc. 122, 4921 (2000).
F. Mohandes, F. Davar, and M. Salavati-Niasari: Magnesium oxide nanocrystals via thermal decomposition of magnesium oxalate. J. Phys. Chem. Solids 71, 1623 (2010).
M. Sharma and P. Jeevanandam: Synthesis of magnesium oxide particles with stacks of plates morphology. J. Alloys Compd. 509, 7881 (2011).
T. Lopez, I. Garcia-Cruz, and R. Gomez: Synthesis of magnesium oxide by the sol-gel method: Effect of the pH on the surface hydroxylation. J. Catal. 127, 75 (1991).
Y.X. Li and K.J. Klabunde: Nanoscale metal oxide particles as chemical regents. Destructive adsorption of a chemical simulant, dimethyl methyl phosophonate, on heat-treated magnesium oxide. Langmuir 7, 1388 (1991).
J.V. Stark, D.G. Park, I. Lagadic, and K.J. Klabunde: Nanoscale metal oxide particles/clusters as chemical reagents. Unique surface chemistry on magnesium oxide as shown by enhanced adsorption of acid gases (sulfur dioxide and carbon dioxide) and pressure dependence. Chem. Mater. 8, 1904 (1996).
G. Duan, X. Yang, J. Chen, G. Huang, L. Lu, and X. Wang: The catalytic effect of nanosized MgO on the decomposition of ammonium perchloride. Powder Technol. 172, 27 (2007).
F. Chen, J. Zhao, and H. Hidaka: Highly selective deethylation of rhodamin B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 5, 209 (2003).
Y. Hu, C. Li, F. Gu, and Y. Zhao: Facile flame synthesis and photoluminescent properties of core/shell TiO2/SiO2 nanoparticles. J. Alloys Compd. 432, L5 (2007).
J. Fang, X. Bi, D. Si, Z. Jiang, and W. Huang: Spectroscopic studies of interfacial structures of CeO2–TiO2 mixed oxides. Appl. Surf. Sci. 253, 8952 (2007).
D. Das, H.K. Mishra, K.M. Parida, and A.K. Dalai: Preparation, physicochemical characterization and catalytic activity of sulfated ZrO2–TiO2 mixed oxides. J. Mol. Catal. A: Chem. 189, 271 (2002).
B. Pal, M. Sharon, and G. Nogami: Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Mater. Chem. Phys. 59, 254 (1999).
J. Lin and J.C. Yu: An investigation on photocatalytic activities of mixed TiO2-rare earth oxides for the oxidation of acetone in air. J. Photochem. Photobiol., A 116, 63 (1998).
J.R. Osman, J.A. Crayston, A. Pratt, and D.T. Richens: Sol-gel processing of IrO2–TiO2 mixed metal oxides based on an iridium acetate precursor. J. Sol-Gel Sci. Technol. 46, 126 (2008).
Y.S. Jung, K.H. Kim, T.Y. Jang, Y. Tak, and S.H. Baeck: Enhancement of photocatalytic properties of Cr2O3-TiO2 mixed oxides prepared by sol-gel method. Curr. Appl. Phys. 11, 358 (2011).
S. Barison, S. Daolio, M. Fabrizio, and A.D. Battisti: Surface chemistry study of RuO2/IrO2/TiO2 mixed-oxide electrodes. Rapid Commun. Mass Spectrom. 18, 278 (2004).
B.M. Reddy, I. Ganesh, and A. Khan: Preparation and characterization of In2O3–TiO2 and V2O5/In2O3–TiO2 composite oxides for catalytic applications. Appl. Catal., A 248, 169 (2003).
B. Julian-Lopez, M. Martos, N. Ulldemolins, J.A. Odriozola, E. Cordoncillo, and P. Escribano: Self-assembling of Er2O3–TiO2 mixed oxide nanoplatelets by template-free solvothermal route. Chem. Eur. J. 15, 12426 (2009).
J.R. Osman, J.A. Crayston, A. Pratt, and D.T. Ritcher: RuO2–TiO2 mixed oxides prepared by hydrolysis of metal alkoxides. Mater. Chem. Phys. 110, 256 (2008).
L.R. Hou, C.Z. Yuan, and Y. Peng: Synthesis and photocatalytic property of SnO2/TiO2 nanotubes composite. J. Hazard. Mater. 139, 310 (2007).
J. Bandara and U.W. Pradeep: Tuning of the flat-band potentials of nanocrystalline TiO2 and SnO2 particles with an outer-shell MgO layer. Thin Solid Films 517, 952 (2008).
K.T. Ranjit, I. Martyanov, D. Demydov, S. Uma, S. Rodrigues, and K.J. Klabunde: A review of the chemical manipulation of nanomaterials using solvent: Gelation-dependent structures. J. Sol-Gel Sci. Technol. 40, 335 (2006).
W. Wei, H. Li, S. Chen, C. Yuan, and Q. Yuan: One step synthesis of ZnO–MgO core-sheath structures. Cryst. Res. Technol. 44, 861 (2009).
M.A. Aramendia, V. Borau, C. Jimenez, J.M. Marinas, A. Porras, and F.J. Urbano: Synthesis and characterization of MgO–B2O3 mixed oxides prepared by coprecipitation; selective dehydrogenation of propan-2-ol. J. Mater. Chem. 9, 819 (1999).
B.M. Reddy, M.V. Kumar, and K.J. Ratnam: Selective oxidation of p-methoxytoluene p-methoxybenzaldehyde over V2O5/CaO–MgO catalysts. Appl.Catal., A 181, 77 (1999).
S. Qiujie, L. Ning, and L. Yi: Preparation of MgO-supported Cu2O catalyst and its catalytic properties for cyclohexanol dehydrogenation. Chin. J. Catal. 28, 57 (2007).
M.E. Martin, R.M. Narske, and K.J. Klabunde: Mesoporous metal oxides formed by aggregation of nanocrystal. Behavior of aluminum oxide and mixtures with magnesium oxide in destructive adsorption of the chemical warfare surrogate 2-chloroethylethyl sulfide. Microporous Mesoporous Mater. 83, 47 (2005).
M.B. Gawande, P.S. Branco, K. Parghi, J.J. Shrikhande, R.K. Pandey, C.A.A. Ghumman, N. Bundaleski, O.M.N.D. Teodoro, and R.V. Jayaram: Synthesis and characterization of versatile MgO–ZrO2 mixed metal oxide nanoparticles and their applications. Catal. Sci. Technol. 1, 1653 (2011).
E.V. Ilian, I.V. Mishakov, A.A. Vedyagin, A.F. Bedilo, and K.J. Klabunde: Synthesis of nanocrystalline VOx/MgO aerogel and their application for destructive adsorption of CF2Cl2. NSTI-Nanotech. 1, 452 (2010).
H. Abimanyu, B.S. Ahn, C.S. Kim, and K.S. Yoo: Preparation and characterization of MgO–CeO2 mixed oxide catalysts by modified coprecipitation using ionic liquids for dimethyl carbonate synthesis. Ind. Eng. Chem. Res. 46, 7936 (2007).
M.E. Llanos, T. Lopez, and R. Gomez: Determination of surface homogeneity of MgO–SiO2 sol-gel mixed oxides by means of CO2 and ammonia thermodesorption. Langmuir 13, 974 (1997).
C.L. Carnes, P.N. Kapoor, K.J. Klabunde, and J. Bonevich: Synthesis, characterization, and adsorption studies of nanocrystalline aluminum oxide and a bimetallic nanocrystalline aluminum oxide/magnesium oxide. Chem. Mater. 14, 2922 (2002).
H. Sieger, J. Suffner, H. Hahn, A.R. Raju, and G. Miehe: Thermal stability of nanocrystalline Sm2O3 and Sm2O3-MgO. J. Am. Ceram. Soc. 89, 979 (2006).
M.A. Aramendia, V. Borau, C. Jimenez, A. Marinas, J.M. Marinas, J.A. Navio, J.R. Ruiz, and F.J. Urbano: Synthesis and textural-structural characterization of magnesia, magnesia-titania, and magnesia-zirconia catalysts. Colloids Surf., A 234, 17 (2004).
H.S. Jung, J.K. Lee, M. Nastasi, S.W. Lee, J.Y. Kim, J.S. Park, K.S. Hong, and H. Shin: Preparation of nanoporous MgO-coated TiO2 nanoparticles and their application to the electrode of dye-sensitized solar cells. Langmuir 21, 10332 (2005).
N. Stubicar, A. Tonejc, and M. Stubicar: Microstructural evolution of some MgO–TiO2 and MgO–Al2O3 powder mixtures during high-energy ball milling and post-annealing studied by x-ray diffraction. Alloys Compd. 370, 296 (2004).
D. Osabe, H. Seyama, and K. Maki: Evaluation of crystallinity in TiO2 films with mixed structures grown on MgO (001) substrates by argon-ion beam sputtering based on infrared reflection-absorption spectra. Appl. Opt. 41, 739 (2001).
J. Bernard, F. Belnou, D. Houidvet, and J.M. Haussonne: Synthesis of pure MgTiO3 by optimizing mixing/grinding condition of MgO + TiO2 powders. J. Mater. Process. Technol. 199, 150 (2008).
T. Lopez, J. Hernandez, R. Gomez, X. Bokhimi, J.L. Boldu, E. Munoz, O. Novaro, and A. Garcia-Ruiz: Synthesis and characterization of TiO2–MgO mixed oxides prepared by the sol-gel method. Langmuir 15, 5689 (1999).
J. Bandara, C.C. Hadapangoda, and W.G. Jayasekera: TiO2/MgO composite photocatalyst: The role of MgO in photoinduced charge carrier separation. Appl. Catal., B 50, 83 (2004).
Z. Wen, X. Yu, S.T. Tu, J. Yan, and E. Dahlquist: Biodiesel production from waste cooking oil catalyzed by TiO2–MgO mixed oxides. Bioresour. Technol. 101, 9570 (2010).
T. Lopez, J. Hernandez-Ventura, D.H. Aguilar, and P. Quintana: Thermal phase stability and catalytic properties of nanostructured TiO2-MgO sol-gel mixed oxides. J. Nanosci. Nanotechnol. 8, 6608 (2008).
S. Utamapanya, K.J. Klabunde, and J.R. Schlup: Nanoscale metal oxide particles/clusters as chemical reagents. Synthesis and properties of ultrahigh surface area magnesium hydroxide and magnesium oxide. Chem. Mater. 3, 175 (1991).
H.K. Kammler, L. Mädler, and S.E. Pratsinis: Flame synthesis of nanoparticles. Chem. Eng. Technol. 24, 583 (2001).
G.W. Jones, B. Lewis, and H. Seaman: The flame temperature of methane-oxygen, methane-hydrogen and methane-acetylene with air. J. Am. Chem. Soc. 53, 3992 (1931).
D. Urzica and E. Gutheil: Structure of laminar methane/nitrogen/oxygen, methane/oxygen and methane/liquid oxygen counterflow flames for cryogenic conditions and elevated pressures. Z. Phys. Chem. 223, 651, (2009).
H.J. Fissan: Temperature distribution in open oxygen air methane-oxygen flame. Combust. Flame 17, 355 (1971).
K.M. Shrestha, C.M. Sorensen, and K.J. Klabunde: Synthesis of CuO nanorods, reduction of CuO into Cu nanorods, and diffuse reflectance measurements of CuO and Cu nanomaterials at near infrared region. J. Phys. Chem. C 114, 14368 (2010).
A.W. Czanderna, C.N.R. Rao, and J.M. Honig: The anatase-rutile transition part 1.— Kinetics of the transformation of pure anatase. Trans. Faraday Soc. 54, 1069 (1958)
J.S.J. Hargreaves, G.J. Hutchings, R.W. Joyner, and C.J. Kiely: The relationship between catalysts morphology and performance in oxidative coupling of methane. J. Catal. 135, 576 (1992).
M. Che and A.J. Tench: Characterization and reactivity of molecular oxygen species on oxide surfaces. Adv. Catal. 32, 1 (1983).
L. Zhang, H. Ji, Y. Lei, and W. Xiao: Oxygen adsorption on anatase surfaces and edges. Appl. Surf. Sci. 257, 8402 (2011).
M. Anpo, Y. Yamada, and Y. Kubokawa: Photoluminescence and photocatalytic activity of MgO powders with coordinatively unsaturated surface ions. J. Chem. Soc. Commun. 50, 714 (1986).
G. Pacchioni and A.M. Ferrari: Surface reactivity of MgO oxygen vacancies. Catal. Today 50, 533(1999).
W.R. Smith and D.G. Ford: Adsorption studies on heterogeneous titania and homogeneous carbon surfaces. J. Phys. Chem. 69, 3587 (1965).
Z. Ding, G.Q. Lu, and P.F. Greenfield: Role of the crystallite phase of TiO2 in heterogeneous photocatalysis for phenol oxidation in water. J. Phys. Chem. B 104, 4815 (2000).
Acknowledgments
The partial financial support of the Department of Energy (DF-FGO2-10ER16202) and KSU Targeted Excellence are acknowledged with gratitude.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Supplementary materials can be viewed in this issue of the Journal of Materials Research by visiting u]http://journals.cambridge.org/jmr.
Rights and permissions
About this article
Cite this article
Shrestha, K.M., Sorensen, C.M. & Klabunde, K.J. MgO–TiO2 mixed oxide nanoparticles: Comparison of flame synthesis versus aerogel method; characterization, and photocatalytic activities. Journal of Materials Research 28, 431–439 (2013). https://doi.org/10.1557/jmr.2012.288
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2012.288