Abstract
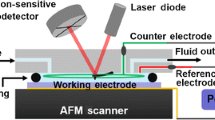
Considerable interest in understanding interfacial phenomena occurring across nanostructured solid oxide fuel cell (SOFC) membrane electrode assemblies has increased demand for in situ characterization techniques with higher resolution. We briefly outline recent advancements in atomic force microscopy (AFM) instrumentation and subsystems in realizing real time imaging at high temperatures and ambient pressures, and the use of these in situ, multi-stimuli probes in collecting local information related to physical and fundamental processes. Here we demonstrate direct probing of local surface potential gradients related to the ionic conductivity of yttria-stabilized zirconia (YSZ) within symmetric SOFCs under intermediate operating temperatures (500–600 °C) via variable temperature scanning surface potential microscopy (VT-SSPM). The conductivity values obtained at different temperatures are then used to estimate the activation energy. These locally collected conductivity and activation energy values are subsequently compared to macroscopic electrochemical impedance results and bulk literature values, thus supporting the validity of the approach.






Similar content being viewed by others
References
A. Atkinson, S. Barnett, R.J. Gorte, J.T.S. Irvine, A.J. McEvoy, M. Mogensen, S.C. Singhal, and J. Vohs: Advanced anodes for high-temperature fuel cells. Nat. Mater. 3, 17 (2004).
S. Park, J.M. Vohs, and R.J. Gorte: Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 404, 265 (2000).
Z. Shao, S.M. Haile, J. Ahn, P.D. Ronney, Z. Zhan, and S.A. Barnett: A thermally self-sustained micro solid-oxide fuel-cell stack with high power density. Nature 435, 795 (2005).
D. Han, X. Liu, F. Zeng, J. Qian, T. Wu, and Z. Zhan: A micro-nano porous oxide hybrid for efficient oxygen reduction in reduced-temperature solid oxide fuel cells. Sci. Rep. 2, 1 (2012).
T.H. Etsell and S.N. Flengas: Electrical properties of solid oxide electrolytes. Chem. Rev. 70, 339 (1970).
J.B. Goodenough: Oxide-ion electrolytes. Annu. Rev. Mater. Res. 33, 91 (2003).
R.M. Ormerod: Solid oxide fuel cells. Chem. Soc. Rev. 32, 17 (2002).
J.W. Fergus: Electrolytes for solid oxide fuel cells. J. Power Sources 162, 30 (2006).
J. Garcia-Barriocanal, A. Rivera-Calzada, M. Varela, Z. Sefrioui, E. Iborra, C. Leon, S.J. Pennycook, and J. Santamaria: Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 321, 676 (2008).
H. Huang, M. Nakamura, P. Su, R. Fasching, Y. Saito, and F.B. Prinz: High-performance ultrathin solid oxide fuel cells for low-temperature operation. J. Electrochem. Soc. 154, 20 (2007).
M. Li, M.J. Pietrowski, R.A.D. Souza, H. Zhang, I.M. Reaney, S.N. Cook, J.A. Kilner, and D.C. Sinclair: A family of oxide ion conductors based on the ferroelectric perovskite Na0.5Bi0.5TiO3. Nat. Mater. 13, 31 (2014).
Z. Wang, M. Cheng, Z. Bia, Y. Dong, H. Zhang, J. Zhang, Z. Feng, and C. Li: Structure and impedance of ZrO2 doped with Sc2O3 and CeO2. Mater. Lett. 59, 2579 (2005).
C. Xia and M. Liu: Low-temperature SOFCs based on Gd0.1Ce0.9O1.95 fabricated by dry pressing. Solid State Ionics 144, 249 (2001).
R.P. O’Hayre, S-W. Cha, W.G. Colella, and F.B. Prinz: Fuel Cell Fundamentals, 2nd ed. (Wiley, New York, 2009).
J. Nielsen and J. Hjelm: Impedance of SOFC electrodes: A review and a comprehensive case study on the impedance of LSM: YSZ cathodes. Electrochim. Acta 115, 31 (2014).
C. Zhang, M.E. Grass, A.H. McDaniel, S.C. DeCaluwe, F.E. Gabaly, Z. Liu, K.F. McCarty, R.L. Farrow, M.A. Linne, Z. Hussain, G.S. Jackson, H. Bluhm, and B.W. Eichhorn: Measuring fundamental properties in operating solid oxide electrochemical cells by using in situ x-ray photoelectron spectroscopy. Nat. Mater. 9, 944 (2010).
S. Kaya, H. Ogasawarab, L-Å. Näslundb, J-O. Forsellc, H.S. Casalongue, D.J. Miller, and A. Nilsson: Ambient-pressure photoelectron spectroscopy for heterogeneous catalysis and electrochemistry. Catal. Today 205, 101 (2013).
A. Kumar, D. Leonard, S. Jesse, F. Ciucci, E.A. Eliseev, A.N. Morozovska, M.D. Biegalski, H.M. Christen, A. Tselev, E. Mutoro, E.J. Crumlin, D. Morgan, Y. Shao-Horn, A. Borisevich, and S.V. Kalinin: Spatially resolved mapping of oxygen reduction/evolution reaction on solid-oxide fuel cell cathodes with sub-10 nm resolution. ACS Nano 7, 3808 (2013).
A. Kumar, S. Jesse, A. Morozovska, E. Eliseev, A. Tebano, N. Yang, and S.V. Kalinin: Variable temperature electrochemical strain microscopy of Sm-doped ceria. Nanotechnology 24, 145401 (2013).
J. Hou, S.S. Nonnenmann, W. Qin, and D.A. Bonnell: A transition in mechanisms of size dependent electrical transport at nanoscale metal-oxide interfaces. Appl. Phys. Lett. 103, 252106 (2013).
B. Cappella and G. Dietler: Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 34, 1 (1999).
J. Zhu, L. Lu, and K. Zeng: Nanoscale mapping of lithium-ion diffusion in a cathode within an all-solid-state lithium-ion battery by advanced scanning probe microscopy techniques. ACS Nano 7, 1666 (2013).
R. Saive, M. Scherer, C. Mueller, D. Daume, J. Schinke, M. Kroeger, and W. Kowalsky: Imaging the electric potential within organic solar cells. Adv. Funct. Mater. 23, 5854 (2013).
J. Broekmaat, A. Brinkman, D.H.A. Blank, and G. Rijnders: High temperature surface imaging using atomic force microscopy. Appl. Phys. Lett. 92, 043102 (2008).
K.V. Hansen, Y. Wu, T. Jacobsen, M.B. Mogensen, and L.T. Kuhn: Improved controlled atmosphere high temperature scanning probe microscope. Rev. Sci. Instrum. 84, 073701 (2013).
S.S. Nonnenmann and D.A. Bonnell: Miniature environmental chamber enabling in situ scanning probe microscopy within reactive environments. Rev. Sci. Instrum. 84, 073707 (2013).
S.S. Nonnenmann, R. Kungas, J. Vohs, and D.A. Bonnell: Direct in situ probe of electrochemical processes in operating fuel cells. ACS Nano 7, 6330 (2013).
T-H. Yeh, W-C. Hsu, and C-C. Chou: Mechanical and electrical properties of ZrO2 (3Y) doped with RENbO4 (RE = Yb, Er, Y, Dy, YNd, Sm, Nd). J. Phys. IV France 128, 213 (2005).
M. Han, X. Tang, H. Yin, and S. Peng: Fabrication, microstructure and properties of a YSZ electrolyte for SOFCs. J. Power Sources 165, 757 (2007).
J.M. Vohs and R.J. Gorte: High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 21, 943 (2009).
R. Küngas, J.M. Vohs, and R.J. Gorte: Systematic studies of the cathode-electrolyte interface in SOFC cathodes prepared by infiltration. ECS Trans. 35, 2085 (2011).
Y. Huang, J.M. Vohs, and R.J. Gorte: Fabrication of Sr-doped LaFeO3 YSZ composite cathodes. J. Electrochem. Soc. 151, A646 (2004).
R. Küngas, J.M. Vohs, and R.J. Gorte: Effect of the ionic conductivity of the electrolyte in composite SOFC cathodes. J. Electrochem. Soc. 158, B743 (2011).
R. Küngas, A.S. Yu, J. Levine, J.M. Vohs, and R.J. Gorte: An investigation of oxygen reduction kinetics in LSF electrodes. J. Electrochem. Soc. 160, F205 (2013).
W.G. Bessler, S. Gewies, and M. Vogler: A new framework for physically based modeling of solid oxide fuel cells. Electrochim. Acta 53, 1782 (2006).
S.R. Hui, J. Roller, S. Yick, X. Zhang, C. Decès-Petit, Y. Xie, R. Maric, and D. Ghosh: A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J. Electrochem. Soc. 172, 493 (2007).
R. Pornprasertsuk, P. Ramanarayanan, C.B. Musgrave, and F.B. Prinz: Predicting ionic conductivity of solid oxide fuel cell electrolyte from first principles. J. Appl. Phys. 98, 103513 (2005).
ACKNOWLEDGMENTS
C.R.P. and D.A.B. were partially supported from the Department of Energy Office of Basic Science DE-FG02-00ER45813-A000 to carry out this research. Facilities used at the Nano/Bio Interface Center were supported through the National Science Foundation NSEC DMR08-32802.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Pérez, C.R., Oh, TS. et al. Probing local electrochemical activity within yttria-stabilized-zirconia via in situ high-temperature atomic force microscopy. Journal of Materials Research 30, 357–363 (2015). https://doi.org/10.1557/jmr.2014.295
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2014.295