Abstract
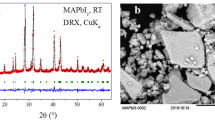
Methylammonium-tin-iodide (MASnxI3, 0.9 ≤ x ≤ 1.4) systems were prepared by self assembly process in aqueous solutions. The “as-prepared” MASnxI3 systems exhibit a crystalline tetragonal structure (space group I4cm) with polyhedral-shaped crystallites. The as-prepared samples were annealed at T = 150 °C, t = 8 h under nitrogen and synthetic air. Under nitrogen, the CH3NH3SnxI3 systems adopted a cubic crystalline structure (space group P4mm) with crystallites of 2–4 µm length, whereas under air, the formation of noncrystalline phases was observed. The optical absorption spectra displayed absorption edges at 1107.0 nm (x = 0.9), 1098.6 nm (x = 1.0), and 1073.2 nm (x = 1.1), respectively, whereas at higher Sn-content (x ≥ 1.2), a broad tail of the absorbance profile was observed. The photoluminescence (PL) emission spectra (RT, λexc = 500 nm) showed major PL-events over 1 µm range and the appearance of additional bands at increasing the Sn-content. The fabrication of layers with a semiconducting behavior was demonstrated.







Similar content being viewed by others
References
M.A. Green, K. Emery, Y. Hishikawa, W. Warta, and E.D. Dunlop: Solar cell efficiency tables (version 48). Prog. Photovoltaics 24, 905–913 (2016).
A. Kojima, K. Teshima, Y. Shirai, and T. Miyasaka: Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
A. Binek, M.L. Petrus, N. Huber, H. Bristow, Y. Hu, T. Bein, and P. Docampo: Recycling perovskite solar cells to avoid lead waste. ACS Appl. Mater. Interfaces 8, 12881–12886 (2016).
T.M. Schmidt, T.T. Larsen-Olsen, J.E. Carle, D. Angmo, and F.C. Krebs: Upscaling of perovskite solar cells: Fully ambient roll processing of flexible perovskite solar cells with printed back electrodes. Adv. Energy Mater. 5, 1500569 (2015).
L. Dimesso, M. Dimamay, M. Hamburger, and W. Jaegermann: Properties of CH3NH3PbX3 (X = I, Br, Cl) powders as precursors for organic/inorganic solar cells. Chem. Mater. 26, 6762–6770 (2014).
L. Dimesso, Y.M. Kim, and W. Jaegermann: Investigation of formamidinium and guanidinium lead tri-iodide powders as precursors for solar cells. Mater. Sci. Eng., B 204, 27–33 (2016).
P.P. Boix, S. Agarwala, T. Ming Koh, N. Mathews, and S.G. Mhaisalkar: Perovskite solar cells: Beyond methylammonium lead iodide. J. Phys. Chem. Lett. 6, 898–907 (2015).
I.R. Benmessaoud, A-L. Mahul-Mellier, E. Horvath, B. Maco, M. Spina, H. Lashuel, and L. Forro: Health hazard of the methylammonium lead iodide based perovskites: Cytotoxicity studies. Toxicol. Res. 5, 407–419 (2016).
H. Needleman: Lead poisoning. Annu. Rev. Med. 55, 209–222 (2004).
C.D. Toscano and T.R. Guilarte: Lead neurotoxicity: From exposure to molecular effects. Brain Res. Rev. 49, 529–554 (2005).
B. Pourrut, M. Shahid, C. Dumat, P. Winterton, and E. Pinelli: Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 213, 113–136 (2011).
Y. Ogomi, A. Morita, S. Tsukamoto, T. Saitho, N. Fujikawa, Q. Shen, T. Toyoda, K. Yoshino, S.S. Pandey, T. Ma, and S. Hayase: CH3NH3SnxPb(1−x)I3 perovskite solar cells covering up to 1060 nm. J. Phys. Chem. Lett. 5, 1004–1011 (2014).
B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D’Haen, L. D’Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca, E. Mosconi, F. De Angelis, and H.G. Boyen: Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv. Energy Mater. 5, 1500477 (2015).
C. Liu, J. Fan, H. Li, C. Zhang, and Y. Mai: Highly efficient perovskite solar cells with substantial reduction of lead content. Sci. Rep. 6, 35705 (2016).
L. Dimesso, C. Das, M. Stoehr, T. Mayer, and W. Jaegermann: Effect of the annealing atmosphere on the properties of cesium tin iodide (CsSnI3) systems. Mater. Chem. Phys. 197, 27–35 (2017).
D. Scaife, P. Weller, and W. Fisher: Crystal preparation and properties of cesium tin(II) trihalides. J. Solid State Chem. 9, 308–314 (1974).
L.S. Foster, H.G. Nahas, and E.E. Lineken: Hydriodic acid: Regeneration of oxidized solutions. In Inorganic Syntheses, Vol. 2, W.C. Fernelius, ed. (John Wiley & Sons, Inc., Hoboken, NJ, USA, 1946).
Y. Dang, Y. Zhou, X. Liu, D. Ju, S. Xia, H. Xia, and X. Tao: Formation of hybrid perovskite tin iodide single crystals by top-seeded solution growth. Angew. Chem., Int. Ed. 55, 3447–3450 (2016).
G.M. Bartenev, I.P. Suzdalev, and A.D. Tsyganov: Mössbauer effect study of the structure of inorganic glasses. Phys. Status Solidi 37, 73–78 (1970).
L. Dimesso, C. Fasel, K. Lakus-Wollny, T. Mayer, and W. Jaegermann: Thermal (in)-stability of lead-free CH3NH3SnxI3 systems (0.9 ≤ x ≤ 1.1) prepared by solution method for photovoltaics. Mater. Sci. Semicond. Process. 68, 152–158 (2017).
D.B. Mitzi: Synthesis, crystal structure, and optical and thermal properties of (C4H9NH3)2MI4 (M = Ge, Sn, Pb). Chem. Mater. 8, 791–800 (1996).
B. Brunetti, C. Cavallo, A. Ciccioli, G. Gigli, and A. Latini: On the thermal and thermodynamic (in)stability of methylammonium lead halide perovskites. Sci. Rep. 6, 31896 (2016).
C.C. Stoumpos, C.D. Malliakas, and M.G. Kanatzidis: Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52, 9019–9038 (2013).
N.K. Noel, S.D. Stranks, A. Abate, C. Wehrenfennig, S. Guarnera, A.A. Haghighirad, A. Sadhanala, G.E. Eperon, S.K. Pathak, M.B. Johnston, A. Petrozza, L.M. Herza, and H.J. Snaith: Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 7, 3061–3068 (2014).
M.G. Ju, G. Sun, Y. Zhaob, and W.Z. Liang: A computational view of the change in the geometric and electronic properties of perovskites caused by the partial substitution of Pb by Sn. Phys. Chem. Chem. Phys. 17, 17679–17687 (2015).
H. Li and Y. Oshima: Elementary reaction mechanism of methylamine oxidation in supercritical water. Ind. Eng. Chem. Res. 44, 8756–8764 (2005).
C. Kittel: Introduction to Solid State Physics, 6th ed., Vol. 185 (John Wiley, New York, USA, 1986).
F. Hao, C.C. Stoumpos, D.H. Cao, R.P.H. Chang, and M.G. Kanatzidis: Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 8, 489–494 (2014).
S.S. Novosad and R.O. Kovalyuk: Absorption, luminescence, and electronic properties of CdI2:Sn2+ crystals. Inorg. Mater. 33, 1183–1188 (1997).
R.A. Howie, W. Moser, and I.C. Trevena: The crystal structure of tin(II) iodide. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 28, 2965–2971 (1972).
ACKNOWLEDGMENTS
Many thanks are owed to Mr. J-C. Jaud for XRD technical assistance, to Mrs. K. Lakus-Wollny for SEM technical assistance, and to Mrs. C. Fasel for DTA-TG technical assistance. The authors thank the Federal Ministry of Research and Development (BMBF) of Germany (Project “Perosol” No. 03SF0483B) for the financial support during this work.
Author information
Authors and Affiliations
Corresponding author
Supplementary Material
43578_2017_32224132_MOESM1_ESM.doc
Supporting Information: Investigation on the Properties of Hybrid CH3NH3SnxI3 (0.9 ≤ x ≤ 1.4) Perovskite Systems (approximately 6.77 MB)
Rights and permissions
About this article
Cite this article
Dimesso, L., Stöhr, M., Das, C. et al. Investigation on the properties of hybrid CH3NH3SnxI3 (0.9 ≤ x ≤ 1.4) perovskite systems. Journal of Materials Research 32, 4132–4141 (2017). https://doi.org/10.1557/jmr.2017.418
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2017.418