Abstract
Background: Solar UV radiation (UVR) is composed of UVB (290–320 nm) and UVA (320–400 nm) wavelengths. Only two sunscreen active ingredients approved in the US, avobenzone (butylmethoxydibenzoylmethane) and zinc oxide (ZnO), provide true broad-spectrum protection against UVA wavelengths >360 nm. Although effective against shorter UVR wavelengths <360 nm, titanium dioxide (TiO2) is also often believed to confer broad-spectrum protection and is substituted for ZnO or avobenzone. To sustain its absorption capacity within a sunscreen film during UVR exposure, avobenzone needs to be formulated into sunscreen products using sound formulation strategies.
Objectives: To characterize the efficacy of avobenzone, ZnO, and TiO2 in terms of their abilities to provide broad UVA protection and to demonstrate the effectiveness of the different formulation strategies used today to maintain the efficacy of avobenzone even during prolonged exposures to UVR.
Methods: UVA efficacy was assessed by measuring absorbance profiles in vitro using Vitro Skin® (IMS Inc., Orange, CT, USA) as an inert substrate and by determining UVA protection factors (PFA) on human skin. The impact of avobenzone loss on sun protection factor (SPF) and PFA values was evaluated by serially reducing avobenzone concentrations in an otherwise photostable product. The photostabilizing influence of specific formulation ingredients was monitored by measuring the extent to which they prevented UVRinduced degradation of avobenzone, whereas photostability of commercial sunscreen products was quantified by measuring the percentage change in absorbance within the UVB and UVA spectral regions following irradiation of thin product films on inert substrates.
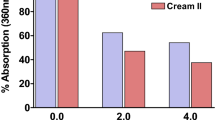
Results: Model formulations containing 3% avobenzone or 5% ZnO provided superior attenuation of UVA wavelengths >360 nm compared with formulas containing 5% TiO2. Additionally, sunscreen products of similar SPF containing avobenzone or ZnO exhibited significantly higher PFA values than those containing TiO2. The addition of photostabilized avobenzone or ZnO increased PFA values nearly 3-fold, whereas the addition of TiO2 increased PFA values only modestly. Judicious selection of sunscreen actives alone or in combination with extra stabilizing agents maintained the photostability of avobenzone in formulations to deliver sustained broad-spectrum absorbance during 4 hours of exposure to UVR. Small losses (<20%) of avobenzone did not significantly reduce a product’s protective effects as measured by SPF and PFA values on human skin.
Conclusions: TiO2 provided neither the same level ofUVA attenuation nor the same degree of UVA protection on human skin as did products containing photostabilized avobenzone or ZnO. Hence, TiO2 cannot be considered a substitute for avobenzone or ZnO in providing high levels of UVA protection to human skin.Use of proper formulation strategies can ensure that avobenzone losses are minimized to the extent that they have no impact on a product’s ability to deliver sustained protection, even over periods of prolonged exposure to UVR.








Similar content being viewed by others
References
Urbach F. The negative effect of solar radiation: a clinical overview. In: Giacomoni PU, editor. Sun protection in man, ESP comprehensive series in photosciences. Vol 3. Amsterdam: Elsevier Sciences, 2001: 41–67
Kaija K, Hanneman DO, Cooper KD, et al. Ultraviolet immunosuppression: mechanisms and consequences. Dermatol Clin 2006; 24: 19–26
Fisher GJ. The pathophysiology of photoaging of the skin. Cutis 2005; 75: 5–9
De Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol 2002; 15: 316–20
DeLeo V. Sunscreen use in photodermatoses. Dermatol Clin 2006; 24: 27–33
Sander CS, Chang H, Salzman S, et al. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol 2002; 118: 618–25
Vielhaber G, Grether-Beck S, Koch O, et al. Sunscreens with an absorption maximum of ! 360nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1 and interleukin-6 in human dermal fibroblast. Photochem Photobiol Sci 2006; 5: 275–82
Schroeder P, Gremmel T, Berneburg M, et al. Partial depletion of mitochondrial DNA from human skin fibroblasts induces a gene expression profile reminiscent of photoaged skin. J Invest Dermatol 2008; 128: 2297–303
Haliday GM, Agar NS, Barnetson RSC, et al. UVA fingerprint mutations in human skin cancer. Photochem Photobiol 2005; 81: 3–8
Runger TM. C-T transition mutations are not solely UVB-signature mutations, because they are also generated by UVA. J Invest Dermatol 2008; 128: 2138–40
Sander SS, Hong C, Hamm F, et al. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol 2004; 43: 326–35
Sayre RM, Dowdy JC, Lott DL, et al. Commentary on ‘UVB-SPF’: the SPF labels of sunscreen products convey more than just UVB protection. Photodermatol Photoimmunol Photomed 2008; 24: 218–20
Food and Drug Administration. Sunscreen drug products for over-the-counter use: final monograph. Fed Reg 1999 May 21; 64 (98): 27666–93
Pinnell SR, Fairhurst D, Gillies R, et al. Microfine zinc oxide is a superior sunscreen ingredient to microfine titanium dioxide. Dermatol Surg 2000; 26: 309–13
Cole C. Multicenter evaluation of sunscreen UVA protectiveness with the protection factor test method. J Am Acad Dermatol 1994; 5 (1): 729–36
Food and Drug Administration. Sunscreen drug products for over-the-counter use: amendment to the tentative final monograph, enforcement policy. Fed Regist 1998 Oct 22; 63 (204): 56587
Stanfield J, Edmonds S, Agin P. An evaluation of methods for measuring sunscreen ultraviolet A protection factors. In: Lowe NJ, Shaath NA, Pathak MA, editors. Sunscreens: development, evaluation and regulatory aspects. New York: Marcel Dekker, 1997: 537–58
Beasley DG, Beard J, Stanfield JW, et al. Evaluation of an economical sunlamp that emits a near solar UV power spectrum for conducting photoimmunological and sunscreen immune protection studies. Photochem Photobiol 1996; 64 (2): 303–9
Schwack W, Rudolph T. Photochemistry of dibenzoyl methane UVA filters: part I. J Photochem Photobiol B: Biol 1995; 28: 229–34
Bonda CA. Research pathways to photostable sunscreens. Cosmet Toil 2008; 123 (2): 49–60
Huong SP, Rocher E, Fourneron JD, et al. Photoreactivity of the sunscreen butylmethoxydibenzoylmethane (DBM) under various experimental conditions. J Photochem Photobiol A: Chem 2008; 196: 106–12
Stamatakis P, Palmer BR, Bohren CF, et al. Optimum particle size of titanium dioxide and zinc oxide for attenuation of ultraviolet radiation. J Paint Tech 1990; 62 (789): 95–8
Schlossman D, Shao Y. Inorganic ultraviolet filters. In: Shaath NA, editor. Sunscreens: regulation and commercial development. 3rd ed. Boca Raton (FL): Taylor & Francis, 2005: 239–79
Tarras-Wahlberg N, Stennhagen G, Larko O, et al. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. J Invest Dermatol 1999; 113: 547–53
Deflandre A, Lang G. Photostability assessment of sunscreens: benzylidene camphor and dibenzoylmethane derivatives. Int J Cosmet Sci 1998; 10: 53–62
Diffey B, Stokes RP, Forestier S, et al. Suncare product photostability: a key parameter for a more realistic in vitro efficacy evaluation. Eur J Dermatol 1997; 7: 226–8
Bonda CA. The photostability of organic sunscreen actives: a review. In: Shaath NA, editor. Sunscreens: regulation and commercial development. 3rd ed. Boca Raton (FL): Taylor & Francis, 2005: 321–52
Bonda CA, Steinberg DC. A new photostabilizer for full spectrum sunscreens. Cosmet Toil 2000; 115 (6): 37–45
Turro NJ. Modern molecular photochemistry. Menlo Park (CA): Benjamin/ Cummings Publishing Co., 1978: 329
Maier H, Schauberger B, Brunnhofer K, et al. Change of ultraviolet absorbance of sunscreens by exposure to solar-simulated radiation. J Invest Derm 2001; 117: 256–62
Sayre RM, Dowdy JC. Photostability testing of avobenzone. Cosmet Toil 1999; 114 (5): 85–91
Chaudhuri RK, Lascu Z, Puccetti G, et al. Design of a photostabilizer having built-in antioxidant functionality and its utility in obtaining broad-spectrum sunscreen formulations. Photochem Photobiol 2006; 82: 823–8
Marrot L, Belaidi JP, Lejeune F, et al. Photostability of sunscreen products influences the efficiency of protection with regard to UVR-induced genotoxic or photoaging-related endpoints. Br J Dermatol 2004; 151: 1234–44
Acknowledgments
Donathan G. Beasley and Thomas A. Meyer are employees of Merck Consumer Care. This study was funded by Merck Consumer Care, which was responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Editorial assistance in the preparation of this article was provided by BioScience Communications, New York, NY, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beasley, D.G., Meyer, T.A. Characterization of the UVA Protection Provided by Avobenzone, Zinc Oxide, and Titanium Dioxide in Broad-Spectrum Sunscreen Products. Am J Clin Dermatol 11, 413–421 (2010). https://doi.org/10.2165/11537050-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11537050-000000000-00000