Abstract
The correct use of immunosuppressive drugs has a considerable influence on the prognosis of patients with organ transplants. The appropriate utilisation of the drugs involves the administration of an adequate dosage to reach the blood concentrations that will suppress the alloimmune response, while avoiding secondary toxicities. However, transplanted patients exhibit heterogeneous immunological responses and high inter- and intraindividual pharmacokinetic variabilities. One cause of these variabilities that is rarely considered is circadian rhythms. In vitro and in vivo experiments have clearly demonstrated that all organisms are highly organised according to an internal biological clock that influences various physiological functions. Considering that the absorption, distribution, metabolism and elimination of drugs is influenced by the physiological functions of the body, it is not surprising that the pharmacokinetic, and consequently the pharmacodynamic, profiles of drugs can be influenced by circadian rhythms.
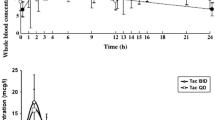
Ciclosporin, a mainstay immunosuppressive drug used following organ transplantation, displays minimum blood concentration (Cmin), maximum blood concentration (Cmax) and area under the blood concentration-time curve (AUC) in the morning that are generally higher than the corresponding parameters in the evening. These observations are supported by the ciclosporin total body clearance and elimination half-life in the morning, which are, on average, higher and shorter, respectively, than those in the evening. In addition, the disposition of tacrolimus is determined by the time of administration. The tacrolimus Cmax and AUC after the morning dose are significantly higher than those after the evening dose.
Finally, the results reported in this review suggest considering more carefully the chronopharmacokinetics of tacrolimus and ciclosporin in order to obtain better results with fewer adverse effects. Significantly, the morning appears to be the best time for therapeutic monitoring using the Cmin, Cmax, concentration at 2 hours after dosing and AUC to modify dosages of tacrolimus and ciclosporin. Less certain are any conclusions about whether, in order to obtain better immunosuppressive control, higher doses must be administered when these drugs are given in the evening to compensate for the higher levels of interleukin-2.



Similar content being viewed by others
References
Krensky AM, Strom TB, Bluestone JA. Immunomodulators: immunosuppressive agents, tolerogens and immunostimulants. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill, 2001: 1463–84
Lechler RI, Sykes M, Thomson AW, et al. Organ transplantation: how much of the promise has been realized? Nat Med 2005; 11: 605–13
Del Tacca M. Prospects for personalized immunosuppression: pharmacologic tools: a review. Transplant Proc 2004; 36: 687–9
Johnston A, Holt DW. Immunosuppressant drugs: the role of therapeutic drug monitoring. Br J Clin Pharmacol 2001; 52: 61S–73S
Reinberg A, Smolensky MH. Circadian changes of drug disposition in man. Clin Pharmacokinet 1982; 7: 401–20
Lemmer B, Bruguerolle B. Chronopharmacokinetics: are they clinically relevant? Clin Pharmacokinet 1994; 26: 419–27
Lemmer B. The clinical relevance of chronopharmacology in therapeutics. Pharmacol Res 1996; 33: 107–15
Levi F. From circadian rhythms to cancer chronotherapeutics. Chronobiol Int 2002; 19: 1–19
Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 2000; 25: 123–8
Hasting MH, Reddy AB, Mayhood ES. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 2003; 4: 649–61
Antoch MP, Song E, Chang AM, et al. Functional identification of the mouse circadian clock gene by transgenic BAC rescue. Cell 1997; 89: 655–67
Turek FW, Allada R. Liver has rhythm. Hepatology 2002; 35: 743–5
Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors and cytokines in rat NK cells. J Immunol 2005; 174: 7618–24
Ishida H, Yamashita C, Kuruta Y, et al. Insulin is a dominant suppressor of sterol 12 alpha-hydroxylase P450 (CYP8B) expression in rat liver: possible role of insulin in circadian rhythm of CYP8B. J Biochem 2000; 127: 57–64
Cannon CP. Circadian variation in the onset of unstable angina and non-Q-wave acute myocardial infarction (the TIMI III Registry and TIMI IIIB). Am J Cardiol 1997; 79: 253–8
Casetta I, Granieri E, Portaluppi F, et al. Circadian variability in hemorrhagic stroke. JAMA 2002; 287: 1266–7
Manfredini R, Poraluppi F, Zamboni P, et al. Circadian variation in spontaneous rupture of abdominal aorta. Lancet 1999; 353: 643–4
Grem JL, Lorrin KY, Venzon DJ, et al. Inter- and intraindividual variation in dihydropyrimidine dehydrogenase activity in peripheral blood mononuclear cells. Cancer Chemother Pharmacol 1997; 40: 117–25
Haus E, Smolensky MH. Biological rhythms in the immune system. Chronobiol Int 1999; 16: 581–622
Born J, Lange TJ, Hansen K, et al. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol 1997; 158: 4454–64
Haus E, Lakatua DJ, Swoyer J, et al. Chronobiology in hematology and immunology. Am J Anat 1983; 168(4): 467–517
Levi F, Halberg F. Circaseptan (about-7-day) dioperiodicity - spontaneous and reactive - and the search for pacemakers. Ric Clin Lab 1982; 12: 323–70
De Vecchi A. Circaseptan rhythmic aspects of rejection in treated patients with kidney transplant. In: Walker CA, Winget CM, Soliman KFA, editors. Chronopharmacology and chemotherapeutics, Tallhassee (FL): A&M University Foundation, 1981: 339–53
Liu T, Cavallini M, Halberg F, et al. More of the need for circadian, circaseptan, and circannual optimization of cyclosporine therapy. Experientia 1986; 42: 20–2
Bowers LD, Canafax J, Singh J, et al. Studies of cyclosporine blood levels: analysis, clinical utility, pharmacokinetics, metabolites, and chronopharmacokinetics. Transplant Proc 1986; 18: 137–43
Sabatè I, Griñó JM, Castelao AM, et al. Diurnal variations of cyclosporine and metabolites in renal transplant patients. Transplant Proc 1990; 22: 1700–1
Ohlman S, Lindholm A, Hagglund H, et al. On the intraindividual variability and chronobiology of cyclosporine pharmacokinetics in renal transplantation. Eur J Clin Pharmacol 1993; 44: 265–9
Milanian I, Ghods AJ, Mahmoudian M, et al. Study of circadian variation of cyclosporine pharmacokinetics. Transplant Proc 1997; 29: 2930–1
Iwahori T, Tacheuchi H, Matsuno N, et al. Pharmacokinetic differences between morning and evening administration of cyclosporine and tacrolimus therapy. Transplant Proc 2005; 37: 1739–40
Baraldo M, Risaliti A, Bresadola F, et al. Circadian variations in cyclosporine C2 concentrations during the first weeks after liver transplantation. Transplant Proc 2003; 35: 1449–51
Châteauvert N, Côté H. Circadian variations in the pharmacokinetics of a new microemulsion formulation of cyclosporine in cardiac transplant recipients. Pharmacother 1998; 18: 364–70
Canafax DM, Cipolle WJM, Hrushesky WJ, et al. The chronopharmacokinetics of cyclosporine and its metabolites in recipients of pancreas allografts. Transplant Proc 1988; 20: 471–7
Min DI, Chen HY, Fabrega A, et al. Circadian variation of tacrolimus disposition in liver allograft recipients. Transplant 1996; 62: 1190–2
Min DI, Chen HY, Lee MK, et al. Time-dependent disposition of tacrolimus and its effect on endothelin-1 in liver allograft recipients. Pharmacother 1997; 17: 457–63
Satoh S, Tada H, Tachiki Y, et al. Chrono and clinical pharmacokinetic study of tacrolimus in continuous intravenous administration. Int J Urol 2001; 8: 353–8
Tada H, Satoh S, Iinuma M, et al. Chronopharmacokinetics of tacrolimus in kidney transplant recipients: occurrence of acute rejection. J Clin Pharmacol 2003; 43: 859–65
Milano G, Chamorey AL. Clinical pharmacokinetics of 5-fluorouracil with consideration of chronopharmacokinetics. Chronobiol Int 2002; 19: 177–89
Harris BE, Song R, He YJ, et al. Circadian rhythm of rat liver dihydropyrimidine dehydrogenase: possible relevance to fluoropyrimidine chemotherapy. Biochem Pharmacol 1998; 37: 4759–62
Harris BE, Song R, Soong SJ, et al. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res 1990; 50: 197–201
Levi FA, Zidani R, Vannetzel JL, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst 1994; 86: 1608–17
Canal P, Squall A, de Forni M, et al. Chronopharmacokinetics of doxorubicin in patients with breast cancer. Eur J Clin Pharmacol 1991; 40: 287–91
Koren G, Ferrazzini GM, Sohl H, et al. Chronopharmacology of methotrexate pharmacokinetics in childhood leukaemia. Chronobiol Int 1992; 9: 434–8
Kelly JG, O’Malley K. Clinical pharmacokinetics of calcium antagonists: an update. Clin Pharmacokinet 1992; 22: 416–33
Lemmer B, Nold G, Behne S, et al. Chronopharmacokinetics and cardiovascular effects of nifedipine. Chronobiol Int 1991; 8: 485–94
Gries JM, Benowitz NL, Verotta D. Chronopharmacokinetics of nicotine. Clin Pharmacol Ther 1996; 60: 385–95
Benowitz NL, Chan K, Denaro CP. Stable isotope method for studying transdermal drug absorption: the nicotine patch. Clin Pharmacol Ther 1991; 50: 286–93
Ohdo S, Nakano S, Ogawa N. Chronopharmacokinetics of valproic acid following constant-rate administration in mice. Chronobiol Int 1991; 8: 35–43
Furlanut M, Benetello P, Mayellaro F, et al. Possible circadian rhythm of diphenylhydantoin metabolism in epileptic patients. Riv Farmacol Ter 1978; 9: 259–66
Labreque G, Belanger PM. Biological rhythms in the absorption, distribution, metabolism and excretion of drugs. Pharmacol Ther 1991; 52: 95–107
Feuers RJ, Scheving LE. Chronobiology of hepatic enzymes. Ann Rev Chronopharmacol 1988; 4: 209–54
Bruguerolle B. Chronopharmacokinetics: current status. Clin Pharmacokinet 1998; 35: 83–94
Halloran PF, Helms MH, Kung L, et al. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplant 1999; 68: 1356–61
Dunn CJ, Wagstaff AJ, Perry CM, et al. Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral) 1 in organ transplantation. Drugs 2001; 61: 1957–2016
Oellerich M, Armstrong VW, Kahan B, et al. Lake Louis Consensus Conference on cyclosporine monitoring in organ transplantation: report of the consensus panel. Ther Drug Monit 1995; 17: 642–54
Nashan B, Bock A, Bosmans JL, et al. Use of neoral C monitoring: a European consensus. Transplant Int 2005; 18: 768–78
Friman S, Backman L. A new microemulsion formulation of cyclosporin: pharmacokinetic and clinical features. Clin Pharmacokinet 1996; 30: 181–93
Bowers LD, Rabatin JT. Circadian pharmacodynamics of cyclosporine in rats and man. In: Reinberg A, Smolensky M, Labrecque G, editors. Annual review of chronopharmacology. Vol. 3. New York: Pergamon Press, 1986: 219–22
Malmary MF, Kabbaj K, Labat C, et al. Chronopharmacokinetics of cyclosporine A in the Wistar rat following oral administration. Eur J Drug Met Pharmacokinet 1992; 17: 135–44
Tredger JM, Chhabra RS. Circadian variations in microsomal drug-metabolizing enzyme activities in rat and rabbit tissue. Xenobiotica 1977; 7: 481–9
Venkataramanan R, Yang S, Burckart GJ, et al. Diurnal variation in cyclosporine kinetics. Ther Drug Monit 1986; 8: 380–1
Heifets M, Cooney GF, Shaw LM, et al. Diurnal variation of cyclosporine clearance in stable renal transplant recipients receiving continuous infusion. Transplant 1995; 60: 1615–7
Gupta SK, Southam MA, Hwang SS, et al. Evaluation of diurnal variation in fentanyl clearance. J Clin Pharmacol 1995; 35: 159–62
Petit E, Milano G, Levi F, et al. Circadian rhythm-varying plasma concentration of 5-fluorouracil during a five-day continuous venous infusion at a constant rate in cancer patients. Cancer Res 1988; 48: 1676–9
Klotz U, Reimann IW. Chronopharmacokinetic study with prolonged infusion of midazolam. Clin Pharmacokinet 1984; 9: 469–74
Queneau P, Decousus H, Ollagnier M, et al. Chronokinetics of ketoprofen administered orally and by continuous venous infusion. Rev Rhum Mal Osteoartic 1985; 52: 403–8
Decousus H, Ollagnier M, Cherrah Y, et al. Chronokinetics of ketoprofen infused intravenously at a constant rate. Annu Rev Chronopharmacol 1986; 3: 312–4
Bruguerolle B, Dupont M, Lebre P, et al. Bupivacaine chronokinetics in man after a peridural constant rate infusion. Annu Rev Chronopharmacol 1988; 5: 223–6
Sanders SW, Bishop AL, Moore JG, et al. Intragastric pH and pharmacokinetics of intravenous ranitidine during sinusoidal and constant-rate infusions. Chronobiol Intern 1991; 8: 267–76
Philip-Joet F, Bruguerolle B, Lagir F, et al. Effects of a constant dose rate of terbutaline on circadian peak expiratory flow, heart rate and systolic arterial pressure in patients with asthma exacerbation. Respiration 1992; 59: 197–200
Levy GA. C2 monitoring strategy for optimising cyclosporin immunosuppression from the Neoral formulation. BioDrugs 2001; 15: 279–90
Scott LJ, McKeage K, Keam SJ, et al. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs 2003; 63: 1247–97
Fujimura A, Shiga T, Ohashi K, et al. Chronopharmacokinetic study of a new immunosuppressive agent, FK 506, in mice. Jpn J Pharmacol 1993; 61: 137–9
Fujimura A, Ebihara A. Administration time-dependent toxicity of a new immunosuppressive agent, tacrolimus (FK506). Life Sci 1994; 55: 485–90
Uchida H, Kobayashi E, Ogino Y, et al. Chronopharmacology of tacrolimus in rats: toxicity and efficacy in a mouse-to-rat intestinal transplant model and its pharmacokinetic profile. Transplant Proc 1999; 31: 2751–3
Yamauchi A, Oishi R, Kataoka Y, et al. Tacrolimus-induced neurotoxicity and nephrotoxicity is ameliorated by administration in the dark phase in rats. Cell Mol Neurobiol 2004; 24: 695–704
Palm S, Postier E, Hinrichsen H, et al. Twenty-four hour analysis of lymphocyte subpopulations and cytokines in healthy subjects. Chronobiol Int 1996; 13: 423–34
Bertouch JV, Roberts-Thomson PJ, Bradley J. Diurnal variation of lymphocyte subsets identified by monoclonal antibodies. BMJ 1983; 286: 1171–2
Oellerich M, Armstrong VW, Schutz E, et al. Therapeutic drug monitoring of cyclosporine and tacrolimus: update on Lake Louise Consensus Conference on Cyclosporin and Tacrolimus. Clin Biochem 1998; 31: 309–16
Chassard D, Brugurolle B. Chronobiology and anesthesia. Anesthesiology 2004; 100: 413–27
Fiorina P, Lattuada G, Slvestrini C, et al. Disruption of nocturnal melatonin rhythm and immunological involvement in ischaemic stroke patients. Scand J Immunol 1999; 50: 228–31
Jung FJ, Yang L, Häer L, et al. Melatonin in vivo prolongs cardiac allograft survival in rats. J Pineal Res 2004; 37: 36–41
Stefanovic V, Ivic M, Mitic M, et al. Diurnal urinary excretion of Cortisol and aldosterone in kidney graft recipients. Pathol Biol 1995; 43: 766–71
Cugini P, Lucia P, Scibilia G, et al. Twenty-four-hour pattern of atrial natriuretic peptide in heart transplantation: evidence for lack of circadian rhythm. Temporal inter-relathionships with plasma rennin activity, aldosterone and Cortisol. Int J Cardiol 1993; 42: 7–14
Armbruster H, Vetter W, Uhlschmid G, et al. Circadian rhythm of plasma renin activity and plasma aldosterone in normal man and in renal allograft recipients. Proc Eur Dial Transplant Assoc 1975; 11: 268–76
Tada H, Satoh S, Iinuma M, et al. Chronopharmacokinetics of tacrolimus in kidney transplant recipients: occurrence of acute rejection. J Clin Pharmacol 2003; 43: 859–65
Fujimura A, Ebihara A. Administration time-dependent toxicity of a new immunosuppressive agent, tacrolimus (FK 506). Life Sci 1994; 55: 485–90
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
The authors thank Dr F. Leita for her assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baraldo, M., Furlanut, M. Chronopharmacokinetics of Ciclosporin and Tacrolimus. Clin Pharmacokinet 45, 775–788 (2006). https://doi.org/10.2165/00003088-200645080-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645080-00002