Abstract
Objectives: (i) To project vaccine parameters, economic consequences and market size associated with HIV-1 vaccination of infants in sub-Saharan Africa through the Expanded Program on Immunisation (EPI); and (ii) to assess threshold values for price and effectiveness.
Study design and methods: Cost-effectiveness analysis using a decision-analysis model linking epidemiological data with economic information. Epidemiological data on the burden of disease of HIVwere obtained from theWorld Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS. The decision analysis model was constructed using estimates of lifetime chances of HIV infection. To assess threshold values for price and effectiveness, a maximum value for cost effectiveness in developing countries of $US100 was used in the base case. One-way and multivariate sensitivity analysis was performed on relevant parameters, assessing the impact of these parameters on the results of our analysis. In the base case, health benefits and consequences were discounted at a rate of 3%.
Study perspective: Societal.
Results: According to our model, introduction of an HIV-1 vaccine in the EPI would result in the vaccination of 8 717 112 infants in sub-Saharan Africa per year. This corresponds to the prevention of 1 839 355 cases of HIV per year, gaining 16 461 800 disability-adjusted life years (DALYs). The cost-effectiveness ratio of the intervention would be $US3.4 per DALY gained (1998 values) at a vaccine price in the base case of $US5. At the same price the estimated size of the market would be approximately $US44 536 111 per year.
Conclusion: If technological and financial problems associated with the development of an HIV vaccine can be solved,HIVvaccination in Africa could be both cost effective and potentially profitable.
Similar content being viewed by others
Since the discovery of the first cases of AIDS in the early 1980s, HIV infection and AIDS have become among the most important health crises of the last century. Nearly 35 million people are now living with HIV, and each year approximately 6 million people become newly infected.[1,2] Of those infected with HIV, 95% are living in developing countries.[2] Because HIV infection typically affects the working part of a country’s population, the impact on the economies of developing countries is severe. Some investigators have stated that HIV will eventually set back the economies of developing countries for decades.[3–5]
Since there is no cure for HIV/AIDS, prevention of transmission is the only way to reduce the number of people who become infected. Until recently, pharmaceutical companies have focused primarily on the development of antiretroviral therapies, giving little attention to vaccine development for African strains of HIV.[6] There are a number of reasons for this. One reason is that vaccine development tends to be more expensive than the development of regular pharmaceuticals, and commercial returns on most vaccines are uncertain.[7] For example, some vaccines such as those against hepatitis B and Haemophilus influenza type b are developed and effective but hardly used in countries with the highest prevalence of these diseases.[8,9] Another reason lies in the typical pathway of vaccine development. After licensure of the vaccine, the vaccine will be manufactured on a small scale and marketed at a high price in developed countries. Later, when initial sales have been successful and research costs recovered, production will be upscaled. Years later, when the vaccine is off patent, it will be introduced at a low price in developing countries.[6,7]
With the highest burden of HIV disease in the developing world, introducing an HIV vaccine in developed countries initially is not optimal; initial introduction should be in developing countries as well. This implicates the need for lower introduction prices. To cover costs for research and development, vaccine developers are then forced to upscale production immediately, causing more financial risks.[6,10] It is clear that this does not stimulate vaccine developers to devote large amounts of resources to HIV vaccine research and development. Several initiatives have recently been proposed to stimulate potential HIV vaccine developers, ranging from vaccine purchase funds to large tax credits.[7,11,12]
Apart from these market difficulties, several other problems have to be overcome before an HIV vaccine can be introduced in the developing world. One of the biggest technological problems lies in the huge genetic diversity of the HIV virus. There are 2 major types of HIV virus, HIV-1 and HIV-2. HIV-1 accounts for most of the infections, while HIV-2 occurs mostly in Angola, Mozambique and certain countries in West Africa.[13] These major types of HIV virus can be subdivided into numerous subtypes. At least 8 subtypes are recognised for HIV-1 while there are at least 6 recognised subtypes for HIV-2. Currently, there is no certainty that a vaccine will be effective against other subtypes than those incorporated in the vaccine. To tackle this problem, a potential HIV vaccine should contain more than one HIV subtype, making the design of the vaccine even more complex. It is also unclear to what extent a vaccine will be effective against small mutations of the virus.[14]
Of all HIV vaccine candidates that are currently in clinical trials, only 2 cover more than 1 HIV-1 subtype;[4] all cover only subtype B and/or E of the HIV-1 virus, the most dominant subtypes of HIV-1 in the industrialised world.[4] As the highest burden of disease in sub-Saharan Africa is caused by other subtypes, it is questionable if these vaccines will show effectiveness in reducing the number of infections in Africa.[15] To date, no candidates for an HIV-2 vaccine exist.[4]
Furthermore, it will also be necessary to develop delivery systems to ensure vaccination of at risk persons. For an HIV vaccination strategy to be successful, it will be necessary to address groups at highest risk of infection, such as intravenous drug users, commercial sex workers and others. These target groups are difficult to reach, especially in developing countries where few healthcare facilities exist. The stigma associated with HIV/AIDS may also form a barrier, causing lower vaccination rates or even discouraging governments from supporting HIV vaccination programmes.[9] Approaching potential vaccine recipients through sexually transmitted disease (STD) clinics, antenatal clinics and secondary schools is probably an effective way to reach those at highest risk.
However, a close look at the HIV interventions that have been implemented in Africa reveals that almost all interventions are small scale.[16] There is little experience in developing countries in expanding interventions to a nationwide scale or even international scale, as needed for a vaccination programme to be successful. For example, if secondary schools are used to target adolescents in developing countries, only 25% would potentially be reached.[16] This coverage rate does not even take into account that a significant portion might refuse to be vaccinated.[17] Although no data are available on the coverage of HIV interventions in STD clinics and antenatal clinics, it is reasonable to suspect even lower coverage rates. Rather than the small-scale targeting of adults, focusing on the vaccination of infants might be a way to achieve a better coverage and long term HIV prevention.
Because most of the vaccination programmes in the developing world are for newborns, it seems attractive to use these existing delivery systems for HIV immunisation. Although it would not directly address those groups at highest risk of infection, the use of these systems would provide an alternative way to introduce HIV vaccination in developing countries.
A potential tool for the administration of an HIV vaccine could therefore be the Expanded Program on Immunisation (EPI) of the World Health Organization (WHO). About 75% of the world’s children receive a set of standard vaccinations through this programme.[18] The EPI vaccination programme consists of vaccination against diphtheria, polio, tetanus, measles, tuberculosis, and pertussis.[18] The vaccines used are mostly off-patent and therefore cheap. It is a very cost effective way to prevent morbidity and mortality caused by these diseases.[19]
To ensure immediate access in the future for those in greatest need of an HIV vaccine, several financial and logistical issues will have to be addressed now. For instance, mechanisms have to be designed to finance vaccine research and development and to design proper delivery mechanisms. Assessments need to be made about the potential size of the market and price per dose of the vaccine. Because the vaccines will be primarily needed in developing countries, the initial price of the vaccine has to be low enough to be affordable. On the other hand, prices will have to be high enough to provide incentives for the pharmaceutical industry to perform research. For governments and pharmaceutical companies, knowledge of the future market and minimal requirements of the vaccine (such as minimal effectiveness) are necessary.
In this paper we aim to provide some of this information. In our analysis we assess vaccine parameters such as vaccine coverage rates and minimal effectiveness, economic consequences and market size of HIV vaccination of infants in sub-Saharan Africa through the EPI using cost-effectiveness analysis techniques.[20] Using cost-effectiveness analysis we project the total costs and consequences of HIV vaccination of infants in sub-Saharan Africa within the EPI. We also assess threshold values for price and effectiveness by using the World Bank standard for cost effectiveness in developing countries.[21]
Materials and Methods
Data Sources and Study Design
Epidemiological data on the burden of disease of HIV were obtained from the WHO and the Joint United Nations Programme on HIV/AIDS (UNAIDS).[2,4,14,22,23] Based on the data, it was not possible to distinguish between HIV-1 and HIV-2. Therefore we excluded Angola, Burkina-Faso, Cape Verde, Gambia, Ghana, Guinea, Guinea-Bissau, Mozambique and Senegal from our analysis; these countries have been reported to have a significant prevalence of HIV-2 infections.[13,14] Because of lack of reliable data, we also had to exclude Comoros, Gabon, Mauritania, Lesotho, the Republic of the Congo, Rawanda, Seychelles and Zambia from our analysis.
We projected vaccination of all newborns with HIV-1 vaccine in the following sub-Saharan countries: Benin, Botswana, Burundi, Cameroon, Central African Republic, Chad, Democratic Republic of Congo, Djibouti, Ethiopia, Kenya, Liberia, Madagascar, Malawi, Mali, Namibia, Niger, Nigeria, Senegal, Sierra Leone, Somalia, Sudan, Swaziland, South Africa, Tanzania, Togo, Uganda and Zimbabwe. Throughout the rest of this paper the abbreviation HIV refers to HIV-1.
The number of surviving newborns in 1998 in each country was used as an estimate of the target population. To estimate the number of vaccinated infants, we used vaccine coverage rates as obtained in these countries with the EPI.[24]
Data from the World Bank regarding cut-off points for acceptable cost-effectiveness ratios (CERs) for health interventions in developing countries were used.[21] To estimate costs for counselling of mothers and administration of vaccine to the infant, data were extracted from other cost-effectiveness studies of HIV interventions in sub-Saharan Africa.[25,26] Only direct costs (see Economic Aspects section) were taken into consideration. The base year for the costs was 1998. Our analysis corresponds with international guidelines for cost-effectiveness analysis.[20]
Incremental costs and threshold values for vaccine parameters were estimated within the framework of a decision-analysis model linking epidemiological data with economic information. The analysis was performed from the societal perspective. The time horizon was 55 years, corresponding with the average life expectancy at birth without the AIDS epidemic in sub-Saharan Africa.[23] Since the major health benefits if vaccination occur in the future, we discounted the health benefits at a rate of 3%.[20]
The Decision Analysis Model
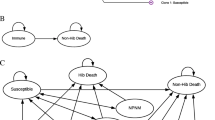
Our model shows the possible pathways of vaccination and associated effects (fig. 1.). The model was constructed using estimates of lifetime chances of acquiring HIV infection combined with epidemiological data (see Epidemiological Data section). We corrected the model for the number of infants born with perinatally acquired HIV infection. To assess the percentage of infants born with perinatally acquired HIV infection, we used the number of mothers infected with HIV attending birth clinics in each country combined with published data on the rate of vertical transmission in sub-Saharan Africa.[22,26] We assumed that coverage rates of vaccination in infants with perinatally acquired HIV infection were equal to those of infants without the disease. Since we did not include therapeutic benefits of vaccination in the model, no health benefits from vaccination were gained in this group. In the base case, we assumed that the vaccine is 60% effective and only needs to be administered once for lifetime protection. In the sensitivity analysis these values were altered.
Epidemiological Data
The size of the target population was 18 559 222 (the number of surviving infants born in 1998 in the specified countries).[24] Data from UNAIDS indicate that 35 to 50% of 15-year-olds in sub-Saharan Africa will eventually die from AIDS.[2,27,28] This information was used together with data on infant mortality to access the lifetime chance of acquiring HIV.
For the period of 1995 to 2000, the average life expectancy at birth without the AIDS epidemic was 54.8 years in sub-Saharan Africa.[23] The mean age of HIV-infected persons was 25 years.[22] Published data indicate a duration of 8 years for moving from asymptomatic HIV to AIDS, and a 1-year progression from AIDS to death[25] i.e. survival after infection with HIV was assumed to be 9 years. Therefore, the average age of death attributable to AIDS was 34 years. The remaining life expectancy at this age without HIV infection was assessed using the life expectancy at birth (infant mortality up to 5 years of age excluded). Mean infant mortality for sub-Saharan Africa in 1998 was 9.2%,[23] leading to an estimated life expectancy for surviving infants at birth without the AIDS epidemic of 60.4 years. This gives an estimated remaining lifetime expectancy at 25 of 26.4 years. However, this figure does not account for mortality occuring between the ages of 5 and 34 years. Therefore, this is likely to be an underestimation of the life expectancy at the age of 34 years. Life years lost were corrected for disability, using disability weights of 0.123 for HIV infection and 0.505 for AIDS.[29]
Table I presents the values for the parameters used in the model.
Economic Aspects
In 1993, the World Bank estimated that adding 2 vaccines to the EPI would increase the costs of the package by 15%.[21] In the base case we assumed that 1 dose of vaccine would be protective i.e. only 1 extra contact would be required. Kremer[12] estimated that the cost of administering 1 extra vaccine would not exceed $US1 per person (1998 values). Costs for counselling of the mothers were assumed to be the same as those in the cost-effectiveness analysis of preventing perinatal transmission by Marseille et al.;[26] thus, the cost was estimated to be $US0.42 per person.
The price per dose of vaccine in the base case was estimated at $US5. This estimate was based loosely on the fact that the hepatitis B vaccine in 1996 retailed in developing countries for approximately $US3.30 for full immunisation (3 doses);[19] $US5 seemed an appropriate price estimate for 1 dose of HIV vaccine in developing countries. The model only calculated direct costs associated with the vaccination of infants with HIV vaccine and the counselling of mothers. It did not account for costs of illness (e.g. medical costs) of HIV/AIDS and indirect costs caused by loss of work productivity. We also performed an analysis on the relationship between price and effectiveness using a fixed outcome for the CER of $US100 per disability-adjusted life year (DALY).
Sensitivity Analysis
Since many of the model parameters are based on assumptions and can vary over time, we performed a sensitivity analysis on parameter values of the model. For example, in the base case we assumed that the lifetime chance at birth of acquiring HIV infection was equal to the lifetime chance of acquiring HIV infection for 15-year-old adolescents. However, it is likely that in reality the lifetime chance of acquiring HIV at birth differs slightly from that at age 15 years; for instance, many people acquire HIV perinatally.
In the sensitivity analysis we examined a wide range of values. The impact of both high and low values for vaccine efficacy on the results was explored. In the base case we used a fixed outcome of $US100 per DALY as a threshold value for cost effectiveness. This ratio reflects the current accepted threshold value for cost effectiveness in developing countries set by the World Bank.[21] Since preferences change over time, we performed a sensitivity analysis exploring a range of values for the fixed outcome of the cost-effectiveness analysis.
In the base case, we discounted health benefits at a rate of 3%. Since the major benefits of HIV vaccination would occur 25 years in the future, there is much uncertainty about the future value of parameters used in the model. To cope with these uncertainties, we discounted DALYs at rates of 0, 5 and 7%.
Since several viral vaccines are only protective after multiple doses (for instance, the hepatitis B vaccine offers maximum protection after 3 doses), the impact of multiple doses of HIV vaccine on the cost effectiveness was assessed. We assumed that 2 additional doses of vaccine would need to be administered to offer full protection. Similar to the hepatitis B vaccine, the first additional dose would need to be administered 1 month after the first dose of the vaccine, while the second additional dose would be given 6 months after the second vaccination. Furthermore, we assumed that vaccine coverage after multiple doses was the same as the coverage after 1 dose of HIV vaccine. This assumption was based on the fact that there is little difference in the coverage rates in sub-Saharan countries of the 1-dose measles vaccine and the diptheria/tetanus/pertussis vaccination which requires 4 doses.[18] Little is known about the price of 1 dose of vaccine; therefore, the impact of different vaccine prices on the cost effectiveness was explored.
In the base case, the remaining average life expectancy at age 34 years in sub-Saharan Africa without the AIDS epidemic was derived from the average life expectancy without the epidemic at birth and corrected for infant mortality. This is not entirely accurate because this value does not take into account the mortality between the ages of 5 and 34 years; the remaining life expectancy at age 34 years would therefore probably be somewhat higher. In the sensitivity analysis we explored the influence of this parameter on the cost effectiveness of the intervention. The effect of varying the average age of infection was also assessed. Using multivariate sensitivity analysis, we explored the impact of changing several model parameters at once.
Results
In the base case, introduction of HIV vaccine through the EPI would result in the vaccination of 8 717 112 infants per year in sub-Saharan Africa. In our model, this would result in the prevention of 1 839 355 cases of HIV per year. HIV vaccination would result in 16 461 800 DALYs gained per year and have a CER of $US3.4 per DALY gained. Figure 2 shows the relationship between price and effectiveness using the fixed ratios for the cost effectiveness of $US25, $US50 and $US100 per DALY.
Results of the sensitivity analysis are presented in table II. The parameters are more sensitive to changes that move the CER up instead of down, suggesting a lower limit for the CER. For instance, decreasing the lifetime chance of acquiring HIV by 71% leads to a more than triple increase in CER, while decreasing the lifetime chance of acquiring HIV by 42% leads to a 30% decrease in CER. If 3 doses of vaccine are needed for protectivity, the CER also almost triples. A 33% decrease in vaccine effectiveness results in a 50% increase of the CER, while a 33% increase in vaccine effectiveness leads to a 25% decrease of the CER. Similar results are seen for the average age of infection and the remaining life expectancy.
Results of the multi-variate sensitivity analysis are shown in figure 3. The most important parameters are the number of doses of vaccine required, the vaccine effectiveness and the lifetime chance of acquiring HIV. The highest CER is found when a 3-dose vaccine with a low effectiveness is used in persons with a low HIV lifetime chance of acquiring HIV. Also, increased CERs are found when a vaccine with low effectiveness is used in persons with a low lifetime chance of acquiring HIV or when a 3-dose vaccine with low effectiveness is used.
Results of the multivariate sensitivity analysis. Low and high refers to the minimum and maximum values for each parameter as used in the sensitivity analysis (see table II). CER = cost-effectiveness ratio; DALY = disability-adjusted life year.
Discussion
It is clear that an effective and safe HIV vaccine is potentially very cost effective. Vaccination is also the only way to slow down and eventually stop the HIV/AIDS epidemic. As described in the introduction, the problems associated with the development of an HIV vaccine are not only technical. Vaccine developers need to be ensured that their investments will have commercial returns. In this study, we assessed the size of the market, using a conservative estimate of the price of vaccine of $US5 per immunisation, at $US44 535 111 per year.
In our analysis, we projected the effects of HIV vaccination of infants. Such an intervention would have considerable impact on the size of the HIV epidemic. However, if we also take vaccination of adults into account, the impact of HIV vaccination would be much larger than projected in this analysis. This would also increase the size of the market tremendously; importantly, it also emphasises the need to establish new systems for the delivery of vaccines. We feel that the benefits of using an existing vaccine delivery system with confirmed high coverage rates, such as the EPI, makes HIV vaccination an interesting long term HIV prevention strategy.
However, to be able to use a vaccine in infants, additional research is necessary to establish safety and immunogenicity in infants. This will take several years; new clinical phase II and III studies will have to be conducted. Another limitation is that the groups at highest risk are not directly targeted. If, however, this intervention is combined with the vaccination of high risk adults, for instance by the vaccination of attenders of STD clinics, antenatal clinics and secondary schools, it might have a major effect on the HIV/AIDS epidemic. As pointed out in our introduction, these systems for delivery of a vaccine are not very effective and need improvement, but may provide a starting point for the intervention.
The vaccination of high risk adults is not only desirable, in that it can break the chain of transmission of the virus in the most effective and immediate way, it also has political benefits. Since the major benefits of an infant HIV vaccination programme would occur 25 years in the future, it may be hard to generate political buy-in for such an intervention. On the other hand, the best way to develop a long term HIV prevention strategy is obviously through childhood vaccination.
In our analysis, we used a static model to calculate the benefits of HIV vaccination. This means that in reality the benefits of the intervention could be greater, because vaccination would also prevent secondary transmission. A large percentage of the population in sub-Saharan Africa is infected with other STDs, causing HIV to be more infectious and persons at risk more vulnerable for infection.[25]
Not much is known about the virological and immunological bases of vaccine-induced protection against HIV. As stated in the introduction, one of the biggest problems with HIV vaccine development lies in the huge genetic diversity of the virus. At the moment it is not clear what the relevance of this will be for the protectivity of a vaccine. Also, since the virus’ mutation rates are very high, there is fear about the occurrence of escape mutants which will be resistant to the immune response triggered by the vaccine. With monotherapy with certain antiretrovirals, for instance nevirapine or lamivudine, the occurrence of highly resistant new viruses can be seen within weeks after the onset of therapy.[30] The relevance of this for vaccine development is also not clear at present. To date, it is also unknown what adverse effects an HIV vaccine will induce and whether the vaccine will show therapeutic effects in patients already infected with the virus. The results of the first phase III trials with a potential HIV vaccine candidate will be presented at the end of 2001 and will hopefully provide a better understanding of the various aspects of HIV vaccine-induced immunology.
One of our main goals was to provide incentives for vaccine manufacturers by estimating the size of the market in sub-Saharan Africa for an HIV vaccine. Even with a conservative assessment, we can state that there is a large initial market for an effective vaccine, if the international society is able to make the necessary arrangements for ensuring proper payment for the efforts of vaccine developers. As stated in the introduction, several international initiatives to tackle this problem are currently under investigation. In the sub-Saharan countries, the mean gross domestic product (GDP) is around $US530.[21] The price of $US5 per vaccine dose that we used as a baseline would have a significant impact on the healthcare budgets of these countries. Therefore, it is clear that a large part of the costs for HIV vaccination will have to be financed by the international community. The establishment of an HIV vaccine purchase fund, as proposed by several organisations,[12] could play a major role in the financing of not only immunisation against HIV, but also against diseases like hepatitis B, pneumonia and H. influenza type B.
The use of different threshold values for the CER shows potential as a tool for policy makers. When the first vaccines are developed, it will be necessary for policy makers to assess the price of a vaccine in relation to its effectiveness; people are probably willing to pay more for a more successful vaccine.[7]
It is likely that first-generation HIV vaccines will have less than optimal effectiveness. Our sensitivity analysis (fig. 3) showed that even a vaccine with a less than 40% effectiveness could have a relevant impact on the HIV epidemic, while maintaining a CER of less than $US50 per DALY. However, a serious ‘adverse effect’ of vaccination could be that vaccine recipients might engage more in high risk behaviours because they believe they are protected against HIV. If a vaccine is only partially effective, this could cause a dramatic increase in the incidence of HIV infection.[31] Even if an HIV vaccine is fully effective, the unintended effects of it on the sexual behaviour of the vaccine recipients could cause dramatic increases in the prevalence of other sexually transmitted diseases such as gonorrhoea, syphilis and hepatitis B. This emphasises the importance of proper counselling of vaccine recipients.
Our analysis incorporated a great number of uncertainties, which could have a significant impact on the results of the study. For instance, little is known about the cost of developing novel viral vaccines; therefore, it is very difficult to predict the cost of 1 dose of vaccine. Even the asking price of $US3.30 for 3 doses (1996 prices) puts the hepatitis B vaccine out of reach for most developing countries. Several new vaccines (for instance the 9-valent conjugated pneumococcal vaccine) retail for prices up to $US100 (1998 values) for multiple doses.[19]
However, because of the severity of the AIDS epidemic, chances are that public pressure will force companies to sell vaccines at affordable prices. Although our model showed sensitivity to the lifetime chance of acquiring HIV and the price of the vaccine, the cost effectiveness of the intervention never exceeded the arbitrary cut-off value for a healthcare intervention set by the World Bank.
Conclusion
We developed a simple model to estimate the cost effectiveness and market size associated with HIV vaccination of infants through the EPI. This study shows that large funds will be necessary not only for developing such a vaccine, but also for executing the vaccination programme. However, it also shows that HIV vaccination of infants in sub-Saharan Africa can potentially be a cost-effective intervention. But before an HIV vaccine is developed and marketed, more research needs to be done to assess the financial and logistical implications of implementing this important intervention.
References
The World Bank Africa Region. Intensifying action against HIV/AIDS in Africa: responding to a development crisis. Washington (DC): The World Bank, 1999
Joint United Nations Programme on HIV/AIDS (UNAIDS). Report on the global HIV/AIDS epidemic. Geneva: UNAIDS, 2000 Jun
International Labour Organisation. The impact of HIV/AIDS on the productive labour force in Africa. Addis Abeba: Eastern Africa Multidisciplinary Advisory Team (EAMAT), 1995: EAMAt working paper No.1
International AIDS Vaccine Initiative (IAVI). Scientific blueprint 2000: accelerating global efforts in AIDS vaccine development. New York (NY): IAVI, 2000 Jul
Jackson H, Kerkhoven R, Lindsey D, et al. HIV/AIDS in southern Africa: the threat to development. London: Russell Press UK for The Catholic Institute for International Relations (CIIR), 1999
World Bank Task Force on the Development of an HIV/AIDS Vaccine for Developing Countries. HIV vaccine industry study Oct–Dec 1998, Draft 20 Mar 2000 [online]. Available from URL: http://www.aien.org [Accessed 2000 Oct 20]
Kremer M. Creating markets for new vaccines: part I: rationale. Harvard Centre for International Development Project on Vaccines (Harvard, MA), Draft: Apr 13, 2000 [online]. Available from URL: http://www.aien.org/vaccine/library.f1ml [Accessed 2000 Oct 20]
Mahoney MT, Ramachandran S, Zhi-Yi X. The introduction of new vaccines into developing countries II: vaccine financing. Vaccine 2000; 18: 2625–35
International AIDS Vaccine Initiative (IAVI). AIDS vaccines for the world: Preparing now to assure access. New York: IAVI, 2000 Jul
Schweitzer SO. Pharmaceutical economics and policy. New York (NY): Oxford University Press, 1997
Smith R. Vaccines and medicines for the world’s poorest: public-private partnerships seem to be essential. BMJ 2000; 320: 952–3
Kremer M. Creating markets for new vaccines: part II: design issues. Harvard Centre for International Development Project on Vaccines (Harvard, MA), Draft: Apr 13, 2000 [online]. Available from URL: http://www.aien.org/vaccine/library.f1ml [Accessed 2000 Oct 20]
Markowitz D. Infection with the human immunodeficiency virus type 2. Ann Intern Med 1993; 118: 211–8
DeVita Jr VT, Hellman S, Rosenberg SA, et al., editors. AIDS: etiology, diagnosis, treatment and prevention. Philadelphia (PA): Lippincott-Raven Publishers, 1997
Essex M. Human immunodeficiency viruses in the developing world. Adv Virus Res 1999; 53: 71–88
Watts C, Kumaranayake L. Thinking big: scaling up HIV-1 interventions in sub-Saharan Africa. Lancet 1999; 354: 1492
Forsythe S. Potential barriers to demand for an AIDS/HIV vaccine in developing countries [online]. Available from URL: http://www.aien.org/vaccine/library.f1ml [Accessed 2000 Oct 20]
World Health Organisation. United Nations Children’s Fund. State of the world’s vaccines and immunisation. Geneva: World Health Organization, 1996 [online]. Available from URL http://www.who.ch [Accessed 2000 Oct 29]
World Health Organization, Department of Vaccines and Biologicals. Vaccines, immunisations and biologicals: 2000–2003 strategy. Geneva: World Health Organization, 2000
Gold MR, Russel LB, Siegel JE, et al., editors. Cost-effectiveness in health and medicine. New York (NY): Oxford University Press, 1996
World Bank. World development report 1993: investing in health. New York (NY): Oxford University Press for the World Bank, 1993
Joint United Nations Programme on HIV/AIDS (UNAIDS)/ World Health Organization Working Group on Global HIV/ AIDS and STI Surveillance. Epidemiological Fact Sheet on HIV/AIDS and sexually transmitted infections: 2000 Update [online]. Available from URL: http://www.unaids.org/hivaidsinfo/statistics/june00/fact_sheets/Africa.html [Accessed 2000 Oct 26]
Population Division Department of Economic and Social Affairs, United Nations. World population prospects: the 2000 revision highlights, Draft. New York (NY): United Nations Population Division, 2001 Feb 28
World Health Organisation Department of Vaccines and Biologicals. WHO vaccine preventable diseases:monitoring system. Geneva: World Health Organisation, 2000
Sweat M, Gregorich S, Sangiwa G, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet 2000; 356: 113–21
Marseille E, Kahn JG, Mmiro F, et al. Cost-effectiveness of single dose nevirapine regimen for mothers and babies to decrease vertical HIV-1 transmission in sub-Saharan Africa. Lancet 1998; 354: 803–9
Whyte B. UNAIDS estimates that half the teenagers in some African countries will die of AIDS. Bull World Health Organ 2000; 78 (7): 946
Joint United Nations Programme on HIV/AIDS (UNAIDS)/ World Health Organisation. AIDS epidemic update. Geneva: UNAIDS/ WHO, 2000 Dec
Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Cambridge (MA): Harvard School of Public Health, 1996
Dalgleish A, Weiss R, editors. HIV and the new viruses. San Diego (CA): Academic Press, 1999
Blower SM, McLean AR. Prophylactic vaccines, risk behaviour change and the probability of eradicating HIV in San Francisco. Science 1994; 265 (5177): 1451–4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bos, J.M., Postma, M.J. The Economics of HIV Vaccines. Pharmacoeconomics 19, 937–946 (2001). https://doi.org/10.2165/00019053-200119090-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200119090-00005