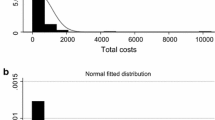
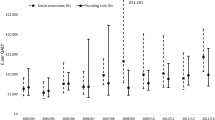
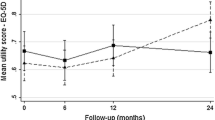
Abstract
Background: Most cost-effectiveness analyses conducted alongside multinational randomized clinical trials (RCT) are carried out applying the unit costs from the country of interest to trial-wide resource use items with the objective of estimating total healthcare costs by treatment group. However, this approach could confound ‘price effects’ with ‘country effects’. An alternative approach is to use multilevel modelling techniques to analyse healthcare resource use (HCRU) from the trial, and obtain country-specific total costs by applying country-specific unit costs to corresponding shrinkage estimates of differential HCRU.
Methods: To illustrate the feasibility of this approach, we analysed data from twin multinational RCTs, which enrolled approximately 2000 individuals into three treatment arms for the management of patients with chronic respiratory disease. The models were implemented using Bayesian multilevel models, to reflect the hierarchical structure of the data while controlling for co-variates at the patient and country level.
Results: This analysis showed that directly modelling the level of HCRU is a promising approach to facilitate cost-effectiveness analyses conducted alongside multinational RCTs, offering several advantages compared with the modelling of direct costs.
Conclusions: It is argued that modelling the level of HCRU within the Bayesian framework avoids confounding the price effects with the country effects and facilitates the estimation of costs for several countries represented in the trial.








Similar content being viewed by others
References
Sculpher MJ, Claxton K, Drummond M, et al. Whither trial-based economic evaluation for health care decision making? Health Econ 2006; 15: 677–87
Sculpher MJ, Pang FS, Manca A, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess 2004; 8: iii–192
Manca A, Willan AR. ‘Lost in translation’: accounting for between-country differences in the analysis of multinational cost-effectiveness data. Pharmacoeconomics 2006; 24 (11): 1101–19
Drummond M, Manca A, Sculpher M. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care 2005; 21: 165–71
Willan AR, Kowgier ME. Cost-effectiveness analysis of a multinational RCT with a binary measure of effectiveness and an interacting covariate. Health Econ 2008; 17: 777–91
Manca A, Rice N, Sculpher MJ, et al. Assessing generalisability by location in trial-based cost-effectiveness analysis: the use of multilevel models. Health Econ 2005; 14: 471–85
Grieve R, Nixon R, Thompson SG, et al. Using multilevel models for assessing the variability of multinational resource use and cost data. Health Econ 2005; 14: 185–96
Grieve R, Nixon R, Thompson SG, et al. Multilevel models for estimating incremental net benefits in multinational studies. Health Econ 2007; 16: 815–26
Nixon RM, Thompson SG. Methods for incorporating covariate adjustment, subgroup analysis and betweencentre differences into cost-effectiveness evaluations. Health Econ 2005; 14: 1217–29
Willke RJ, Glick HA, Polsky D, et al. Estimating countryspecific cost-effectiveness from multinational clinical trials. Health Econ 1998; 7: 481–93
Wordsworth S, Ludbrook A. Comparing costing results in across country economic evaluations: the use of technology specific purchasing power parities. Health Econ 2005; 14: 93–9
Jonsson B, Weinstein MC. Economic evaluation alongside multinational clinical trials: study considerations for GUSTO IIb. Int J Technol Assess Health Care 1997; 13: 49–58
Thompson SG, Nixon RM, Grieve R. Addressing the issues that arise in analysing multicentre cost data, with application to a multinational study. J Health Econ 2006; 25: 1015–28
Cameron AC, Trivedi PK. Regression analysis of count data. New York: Cambridge University Press, 1998
McCullagh P, Nelder J. Generalized linear models, 2nd ed. [monographs on statistics and applied probability]. New York: Chapman and Hall, 1989
Metcalfe C, Thompson SG. The importance of varying the event generation process in simulation studies of statistical methods for recurrent events. Stat Med 2006; 25: 165–79
Gelman A, Carlin J, Stern H, et al. Bayesian data analysis. New York: Chapman and Hall/CRC, 2004
Glick H, Doshi JA, Sonnad SS, et al. Economic evaluation in clinical trials. Oxford: Oxford University Press, 2007
Ohlssen DI, Sharples LD, Spiegelhalter DJ. Flexible random-effects models using Bayesian semi-parametric models: applications to institutional comparisons. Stat Med 2007; 26: 2088–112
Ghosh S, Mukhopadhyay P, Lu J. Bayesian analysis of zero inflated regression models. J Stat Planning Infer 2006; 136: 1360–75
Spiegelhalter D, Thomas A, Best N, et al. Win BUGS user manual: version 1.4. Cambridge: MRC Biostatistics Unit, 2003
Getzen TE. Aggregation and themeasurement of health care costs. Health Serv Res 2006; 41: 1938–54
Rubin D. Multiple imputation for nonresponse in surveys, New York. New York: John Wiley & Sons, 1987
Schafer J. Analysis of incomplete multivariate data. New York: Chapman and Hall, 1997
Martin A. Bayesian inference for heterogeneous event counts. Sociol Methods Res 2003; 31: 30–63
Spiegelhalter D, Best N, Carlin B, et al. Bayesian measures of model complexity and fit: series B (statistical methodology). J R Stat Soc 2002; 64: 583–639
Petrinco M, Pagano E, Desideri A, et al. Information on center characteristics as costs’ determinants in multicenter clinical trials: is modeling center effect worth the effort? Value Health 2008; 12 (2): 325–30
Spiegelhalter D, Abrams K, Miles J. Bayesian approaches to clinical trials and health care evaluation. Hoboken (NJ): John Wiley & Sons, 2004
Sangho M, Jaeun S. Health care utilization among Medicare-Medicaid dual eligibles: a count data analysis. BMC Public Health 2006 1988; 6: 88
Nixon RM, Thompson SG. Parametric modelling of cost data in medical studies. Stat Med 2004; 23: 1311–31
Briggs A, Nixon R, Dixon S, et al. Parametric modelling of cost data: some simulation evidence. Health Econ 2005; 14: 421–8
Conti S, Manca A, Lambert P, et al. Bayesian multivariate modelling of patient level healthcare resource use data in RCTs [abstract]. International Health Economics Association (iHEA); 2007 Jul 9; Copenhagen
Lambert PC, Billingham LJ, Cooper NJ, et al. Estimating the cost-effectiveness of an intervention in a clinical trial when partial cost information is available: a Bayesian approach. Health Econ 2008; 17: 67–81
Moerbeek M. The consequence of ignoring a level of nesting in multilevel analysis. Multivar Behav Res 2004; 39: 149
Wennberg J, Gittelsohn J. Small area variations in health care delivery. Science 1973; 182: 1102–8
Acknowledgements
This study was sponsored by Boehringer Ingelheim International GmbH and conducted by i3 Innovus under contract. Andrea Manca acted as a consultant to i3 Innovus and provided advice on the analysis of the data, the development of the models and the draft of the manuscript. The authors have no conflicts of interest that are directly relevant to this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gauthier, A., Manca, A. & Anton, S. Bayesian Modelling of Healthcare Resource Use in Multinational Randomized Clinical Trials. Pharmacoeconomics 27, 1017–1029 (2009). https://doi.org/10.2165/11314030-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11314030-000000000-00000