Synthesis and Characterization of Hydrogel of Chitosan-Poly (N-Vinyl-2-Pirrolidone) (PVP)- Alginate for Ibuprofen Release
Abstract
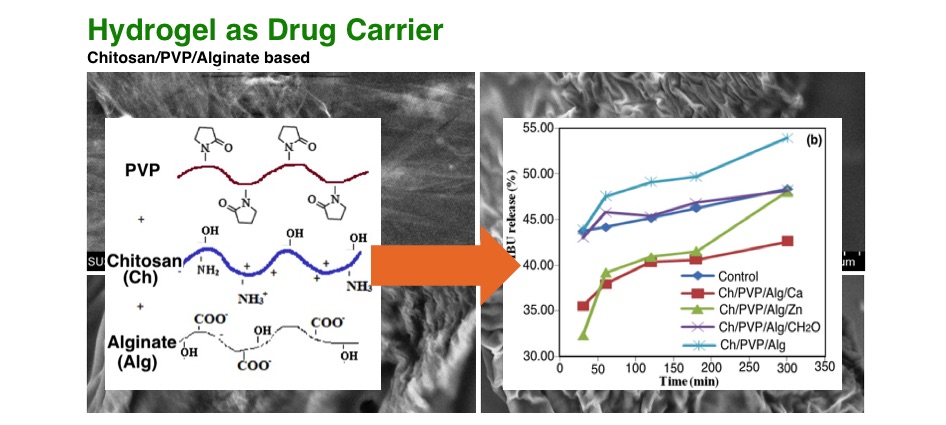
Hydrogels chitosan-poly-(N-vinyl-pyrrolidone)-alginate (Ch/PVP/Alg) have been synthesized with Ca2+, Zn2+ and formaldehyde as crosslinker. Hydrogels with ratio polymer 70:20:10 give a high swelling ratio and good network. The Ch/PVP/Alg/Ca2+ has 463.73% swelling ratio and 80.59% gel. Ch/PVP/Alg/Zn has 489.21% swelling ratio and 81.67% gel. Ch/PVP/Alg crosslinked with formaldehyde result 488.03% swelling ratio and 85.34% gel. Dissolution test of hydrogels in pH 1.2 releases ibuprofen less than 30%. Whereas in the pH 7.4, the release of ibuprofen by hydrogels are relatively high. Ch/PVP/Alg/Ca reach up to 34.63% in 30 minutes and 40.86% for Ch/PVP/Alg/Zn. Meanwhile Ch/PVP/Alg/CH2O can release 44.92% of ibuprofen in 30 minutes. The obtained hydrogel was characterized using infrared (FTIR) spectrophotometry, differential scanning calorimetry (DSC) and scanning electron microscopy (SEM).
References
[1] Aldana, A.A., Gonzalez, A., Strumia, M.C., and Martinelli, M., Mater. Chem. Phys, 2012, 134(1), 317– 324.
[2] Abdel-Mohsen, A.M., Aly, A.S., Hrdina, R., Montaser, A.S., and Hebeish, A., J. Polym. Environ, 2011, 19(4), 1005-1012.
[3] Matricardi, P., Di Meo, C., Coviello, T., Hennink, W.E., and Alhaique, F., Adv. Drug Deliv. Rev, 2013, 65(9), 1172-1187.
[4] Sahoo, S., Sasmal, A., Nanda, R., Phani, A.R., and Nayak, P.L., Carbohydr. Polym, 2010, 79(1), 106-113.
[5] Li, G., Guo, L., Chang, X., and Yang, M., Int. J. Biol. Macromol, 2012, 50(4), 899-904.
[6] Yu, C., Yun-fei, L., Hui-min, T., and Jian-xian, J., Carbohyd. Polym, 2009, 75, 287–292.
[7] Chang, C., Duan, B., Cai, J., and Zhang, L., Eur. Polym. J, 2010, 46(1), 92–100.
[8] Deligkaris, K., Tadele, T.S., Olthuis, W., and Van den Berg, A., Sens. Actuators B. Chem., 2010, 147(2), 765–774.
[9] Zhang, L., Bai, X., Tian, H., Zhong, L., Ma, C., Zhou, Y., Chen, S., and Li, D., Carbohydr. Polym, 2012, 89(4), 1060-1066.
[10] Li, Q., Yang, D., Ma, G., Xu, Q., Chen, X., Lu, F., and Nie, J., Int. J. Biol Macromol, 2009, 44(2), 121-127.
[11] Spagnol, C., Rodrigues, F.H., Pereira, A.G., Fajardo, A.R., Rubira, A.F., and Muniz, E.C., Carbohydr Polym, 2012, 87(3), 2038– 2045.
[12] Zhou, Y., Li, H., Liu, J., Xu, Y., Wang, Y., Ren, H., and Li, X., Polym Adv Technol, 2019, 30(1), 143-152.
[13] Marsano, E., Bianchi, E., Vicini, S., Companino, L., Sionkowka, A., Skopińska, J., and Wiśniewki, W., Polymer, 2005, 46(5), 1595-1600.
[14] Treenate, P., and Monvisade, P., Int. J. Biol. Macromol, 2017, 99, 71-78.
[15] Wu, T., Huang, J., Jiang, Y., Hu, Y., Ye, X., Liu, D., and Chen, J., Food Chem, 2017, 240, 361-369.
[16] Wang, W., and Wang, A., Carbohydr. Polym, 2010, 80(4), 1028–1036.
[17] Kumar, G.P., Phani, A.R., Prasad, R.G.S.V., Sanganal, J.S., Manali, N., Gupta, R., Rashmi, N., Prabhakara, G.S., Salins, C.P., Sandeep, K., Raju, D.B., Int. J. Pharm, 2014, 471(1-2), 146-152.
[18] Guinesi, L.S., and Cavalheiro, E.T.G., Thermochim. Acta, 2006, 444(2), 128-133.
[19] Lewandowska, K., Thermochim. Acta, 2011, 517(1-2), 90-97.
[20] Hao, H., Wang, G. and Sun, J., Drug Met. Rev. 2005, 37(1), 215-234.
[21] Perlman, M.B., Johnson, A.R.N.O.L.D. and Malik, A.B., Am. J. Physiol. Heart Circ. Physiol. 1987, 252(3), H605-H614.
[22] Pire, L.R., Lopes, C.D., Salvador, D., Rocha, D.N. and Pego, A.P., J. Mater. Sci. Mater. Med. 2017, 28(10), 157.
Refbacks

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








