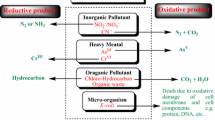
Abstract
The use of redox potential as a control parameter of wastewater treatment processes for characterizing the natural water condition and estimating the antioxidant properties of drinking water has been investigated.
Similar content being viewed by others
References
Voda pytna. Normatyvni dokumenty (Drinking Water. Regulatory Documents), Lviv: NTTs Leonormstandart, vol. 2, 2001.
Nikanorov, A.M., Gidrokhimiya (Hydrochemistry), Leningrad: Gidrometeoizdat, 1989.
Williams, J.B., Williams, L., Baldwin, N., et al., Proc. Nat. Conf. on Environ. Sci. and Technol., (Greensboro, N.C., September 8–10, 2002), Columbus (Ohio): Richland Battelle, 2003.
Tremblay, C.V., Beaubien, A., Charles, P.N., and James, A., Water Sci. and Technol., 1998, vol. 38, no. 6, pp. 121–128.
Wregglessworth, D., Metal Finish, 2004, vol. 102, no. 5, pp. 6–7.
Gottard, W., Patent Application no. 19960275 (Germany), IPC7 G 01 N 27/416, Publ. June 21, 2001.
Nakamura, Sh., Tarasuka, K., and Okuda, A., Patent no. 6235188 (USA), IPC7 C 02 F 1/461, Publ. May 22, 2001.
Khan, S., Patent no. 6340431 (USA), IPC7 B 01 D 17/12, Publ. January 22, 2001.
Blauwitz, U., Patent Application no. 19808412 (Germany), IPC6 G 01 N 33/18, Publ. September 2, 1999.
Navarro, P., Patent no. 6657546 (USA), IPC7 G 08 B 21/00, Publ. December 2, 2003.
Li, X., Qi, J., and Wang, Y., J. Harbin Univ. Giv. Eng. and Archit., 2002, vol. 35, no. 3, pp. 68–70.
Oxidation-Reduction Potential (ORP)/PEDOX, Application Bulletin, Myron L. Company, 2007.
Suslow, T.V., Introduction to ORP as the Standard of Postharvest Water Disinfection Monitoring, US Davis Vegetable Research and Information Center, http://vric.ucdavis.edu/veginfo/foodsafety/orp.pdf.
Bergendahl, J.A. and Stevens, L., Environ. Progress, 2005, vol. 24, no. 2, pp. 214–222.
Shakhmetova, S.G., Bashkir. Khim. Zhurn., 2007, vol. 14, no. 2, pp. 118–120.
Misier, M.O., Eau; ind., nuisances, 2006, no. 288, pp. 57–58.
Bongards, M., Patent Application no. 19702951 (Germany), IPC6 C 02 F 3/30, Publ. July 30, 1998.
Bauman, P., Hansen, J., and Richert, J., KA—Abwasser, Abfal., 2005 vol. 52, no. 12, pp. 1352–1358.
Fuerhacker, M., Bauer, H., Ellinger, R., et al., Chemosphere, 2001, vol. 44, no. 5, pp. 1213–1221.
Gao, D.W., Peng, Y.Z., Liang, H., et al, J. Environ. Sci. and Health, 2003, vol. 38, no. 12, pp. 2933–2942.
Chen, K.C., Chen, C.Y., Peng, J.W., et al., Water Res., 2002, vol. 36, no. 1, pp. 230–238.
Teble, F. and Keiser, D., Umweltpraxis, 2002, vol. 2, no. 4, pp. 35–36.
Reggi, R., World Leather, 1996, vol. 9, no. 7, pp. 47–48.
Dmitrenko, G.N. and Ereshko, T.V., Khimiya i Tekhnologiya Vody, 2005, vol. 27, no. 4, pp. 392–398.
Dmitrenko, G.N., ibid., 2001, vol. 23, no. 3, pp. 329–337.
Imaoki, T., Hirochi, M., Sugiyama, I., et al., Patent Application no. 1038839 EPO, IPC7 C 01 F 1/70, Publ. September 27, 2000.
Gerardi, M.H., Oxidation-Reduction Potential and Wastewater Treatment, New England Interstate Water Pollution Control Commission, Publication and Resources, Interstate Water Report, 2007, http://www.neiwpcc.org/iwr/reduc-tionpotential.asp.
Meierling, L., Wasserwird-Wassertechn., 2003, no. 5, pp. 36–39.
Aizenshtadt, A.M., Bogdanov, M.V., Bogolitsin, K.G., and Abrosimova, A.A., Izv. Vuzov. Lesn. Zhurn., 2006, no. 3, pp. 91–97.
Makar’, A.V., Elektron. Obrabotka Materialov, 2003, no. 2, pp. 80–83, 101.
Meß—und Analysegeräte zum Einsatz in der Wasserverschmutzungskontrolle zunehment gefragt, Galvanotechnik, 2000, vol. 91, no. 5, p. 1430.
Neu bei Dr. Lange: Die ECM—Familie, ibid., 1997, vol. 88, no. 6, p. 2052.
Redox-Industrieregler, ibid., 1999, vol. 90, no. 3, pp. 734–735.
Starkes Wachstum durch neu Leitfähigkeitssensoren, ibid., 2003, vol. 94, no. 2, pp. 402–403.
Datenlogger für Kontrollmessungen, Chem.-Ing.-Technol., 1999, vol. 71, no. 12, pp. 1350.
Analesenmesstechnik für die Wasserraufbereitung, Galvanotechnik, 2005, vol. 96, no. 4, pp. 891.
New patented four-beam turbidity sensor, Int. Environ. Technol., 2001, vol. 11, no. 6, pp. 16.
Oelbner, W., Hermann, S., Schwarz, J., and Koden, H., Chem.-Ing.-Techn., 2000, vol. 72, no. 1/2, pp. 98–101.
Schindler, W., Incom’98, Düsseldorf (Düsseldorf, 1998), 1998.
GOST (State Standard) 2874-82: Woda pit’evaya. Gigienicheskie trebovaniya i kontrol’ za kachestvom (Drinking Water. Hygienic Regulations and Quality Control), Put into effect on January 1, 1984.
Prilutskii, V.I. and Bakhir, V.M., Elektrokhimicheski aktivirovannaya voda: anomal’nye svoistva, mekhanizm biologicheskogo deistviya (Electrochemically Activated Water: Anomalous Properties, the Mechanism of Biological Activity), Moscow, 1997.
Dvornikov, V.M., Tekhnologiya sokhraneniya metastabil’nogo sostoyaniya nizkomineralizovannoi aktivirovannoi vody (Technology of Maintaining the Metastable Condition of Low Mineralized Water), http://www.gepatitu-net.ru/nauch_obos.htm.
Kim, M.J. and Kim, H.K., Life Sci., 2006, no. 79, pp. 2288–2292.
Lee, K.J., Park, S.K., Kim, J.W., et al., J. Int. Soc. Life Inform. Sci., 2004, vol. 22, no. 2, pp. 302–305.
Yasunori, S., Shizuo, K., Akiko, A., et al., Biochem. and Biophys. Res. Commun., 2008, vol. 375, no. 3, pp. 346–350.
Kokichi, H., Dongxu, S., Richard, L., et al., Biophysical Chemistry, 2004, no. 107, pp. 71–82.
Hanaoka, K., J. of Applied Electrochemistry, 2001, no. 31, pp. 1307–1313.
Ikuroh, O., Masahiro, I., Kumiko, T., et al., Nature Medicine, 2007, no. 13, pp. 688–694.
Huang, K, Yan, C, Lee, K., et al., Kidney Int., 2003, no. 64, pp. 704–714.
Yuping, L., Tomohiro, N., Kiichiro, T., et al., Cytotechnol., 2002, no. 40, pp. 139–149.
Dan, J., Sung, H.R., Hyun, W.K., et al., Biosci. Biotechnol. Biochem., 2006, vol. 70, no. 1, pp. 31–37.
Gadek, Z., Li, Y, and Shirahata, S., Animal Cell Technol.: Basic and Appl. Aspects, 2006, no. 3, pp. 377–385.
Kajiyama, S., Hasegawa, G., Asano, M., et al., Nutrition Res., 2008, no. 28, pp. 137–143.
Leonov, B.I., Prilutskii, V.I., and Bakhir, V.M., Fiziko-khimicheskie aspekty biologicheskogo deistviya elektrokhimicheski aktivirovannoi vody (Physico-Chemical Aspects of the Biological Effect of Electrochemically Activated Water), Moscow: VNIIIMT, 1999.
Chetkovic, V.S., Purenovic, J.M., and Jovicevic, J.N., Appl. Clay Sci., 2008, no. 38, pp. 268–278.
Ryuhei, N., Kiichiro, T., Yoshinori, K., et al., Cytotechnol., 2005, no. 47, pp. 97–105.
Yanagira, T., Sato, B., and Syudo, T., Patent no. 2004101402 A (Russia), IPC7 C02F 1/70, 1/30, Publ. in byul. no. 13, May 10, 2005.
Petrushanko, I.Yu. and Lobyshev, V.I., Biofizika, 2001, vol. 46, no. 3, pp. 389–401.
Petrushanko, I.Yu. and Lobyshev, V.I., ibid., 2004, vol. 49, no. 1, pp. 22–31.
Dobosh, D., Elektrokhimicheskie konstanty (Electrochemical Constants), Moscow: Mir, 1980.
Piskarev, I.M., Ushkanov, V.A., Likhachev, P.P., and Myslivets, T.S., Okislitel’no-vosstanovitel’nyi potentsial vody, nasyshchennoi vodorodom (Oxidation-Reduction Potential of Hydrogenated Water), http://zhurnal.ape.relarn.ru/articles/2007/023.pdf.
Shirahata, S., Kabayama, S., Nakano, M., et al., Biochemical and Biophysical Research Communications, 1997, no. 234, pp. 269–274.
Bagley, D., Patent no. 20060273030 (USA), IPC A 23 J 7/00, A 23 J 007/00, Publ. December 7, 2006.
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.V. Goncharuk, V.A. Bagrii, L.A. Mel’nik, R.D. Chebotareva, S.Yu. Bashtan, 2010, published in Khimiya i Tekhnologiya Vody, 2010, Vol. 32, No. 1, pp. 3–19.
About this article
Cite this article
Goncharuk, V.V., Bagrii, V.A., Mel’nik, L.A. et al. The use of redox potential in water treatment processes. J. Water Chem. Technol. 32, 1–9 (2010). https://doi.org/10.3103/S1063455X10010017
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X10010017