Abstract
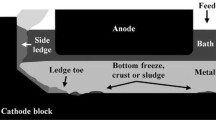
The dynamic behavior (formation/dissolution) of the ledge, depending on the electrolyte overheating temperature, thermal resistance of the lining material, and composite of the cryolite–alumina electrolyte, is studied experimentally using a model installation mimicking the actual conditions of the electrolytic aluminum production. A window that enabled the change of the lining material is mounted into the front wall of the installation case. The ledge formation occurs due to a heat flow formed due to the temperature difference of the electrolyte and electrolyzer walls. The electrolyte cryolite ratio (CR) is varied in a range of 2.1–2.5. The alumina concentration in the electrolyte does not exceed 4.5 wt %. The change in the shape of the working space in the electrolyzer during the electrolysis is determined by the ledge thickness. The active ledge formation in the experimental cell starts upon overheating by 3–4 K. It is shown that a thicker ledge is formed at the same overheating temperature with a decrease in the thermal resistance of the lining material from 16 to 14 m2/W, but a decrease in the thermal resistance in the already formed ledge almost does not affect its thickness. Similarly to the industrial electrolyzer, the ledge profile formed in the experimental cell can be conditionally divided into three zones, notably, bottom ledge, ledge at the metal/electrode interface, and side ledge. The dynamic behavior of the side ledge differs from the bottom ledge; notably, the side ledge is thicker at a high electrolyte CR, while the bottom ledge is thinner. The chemical analysis of components in the dry knock out shows that the CR and Al2O3 concentrations increase over the cell height from top to bottom. It is concluded that the side ledge has a heterogeneous composition that depends on the electrolyte composition and cooling rate.








Similar content being viewed by others
REFERENCES
Sizyakov, V.M., Feshchenko, R.Yu., Bazhin, V.Yu., Patrin, R.K., and Sizyakov, V.M., Features of the high-current electrolyzer bottom destruction, Novye Ogneupory, 2013, no. 5, pp. 5–8.
Yin, E., Liu, Y., Xi, C., and Zhang, J., Developing the GP-320 technology in China, Light Met., 2001, pp. 213–218.
Zeng, S., Analysis of the start-up of Q-350 prebaked aluminium reduction cell, Light Met., 2006, pp. 271–275.
Kvande, H., Preheating, start-up and early operation of Hall-Heroult cells, in 10th Int. course on process metallurgy of aluminium, Trondheirn, Norway, 1991, pp. 15–48.
Sorlie, M. and Oye, H., Cathodes in Aluminium Electrolysis, Düsseldorf: Aluminum-Verlag Marketing & Kommunikation, 2010, 3rd ed.
Vallea, A. and Lenis, V., Prediction of ledge profile in Hall-Heroult cells, Light Met., 1995, pp. 309–313.
Thonstad, J. and Rolseth, S., Equilibrium between bath and side ledge in aluminium cells. Basic principle, Light Met., 1983, pp. 415–425.
Thonstad, J. and Solheim, A., Heat transfer coefficients between bath and side ledge, Light Met., 1983, pp. 425–435.
Solheim, A., Towards the proper understanding of sideledge facing the metal in aluminum cells?Light Met., 2006, pp. 439–443.
Solheim, A., Some aspects of heat transfer between bath and sideledge in aluminum reduction cells, Light Met., 2011, pp. 381–386.
Solheim, A., Rolseth, S., Skybakmoen, E., Stoen, L., Sterten, A., and Store, T., Liquidus temperatures for primary crystallization of cryolite in molten salt systems of interest for the aluminium electrolysis, Met. Trans. B, 1996, vol. 27B, pp. 739–744.
Solheim, A., Crystallization of cryolite and alumina at the metal-bath interface in aluminium reduction cells, Light Met., 2002, pp. 225–230.
Peacey, J.G. and Medlin, G.W., Cell sidewall studies at Noranda Aluminium, Light Met., 1979, pp. 475–480.
Haupin, W.E., Calculating thickness of containing walls frozen from melt, JOM, 1971, vol. 23, no. 7, pp. 41–44.
Danilyuk, I.I., Revisiting the Stefan problem, Usp. Matem. Nauk, 1985, vol. 40, no. 5, pp. 133–185.
Samarskii, A.A. and Vabishchevits, P.N., Vychislitel’naya teploperedacha (Computational Heat Transfer), Moscow: Editorial URSS, 2003.
Sleptsov, S.D. and Rubtsov, N.A., ., Solving the classical single-phase Stefan problem in a modified formulation for semi-transparent media, Prikl. Mekh. Tekh. Fiz., 2013, vol. 54, no. 3, pp. 106–113.
Marios, M., Bertrand, C., Desilets, M., Coulombe, M., and Lacroix, M., Comparison of two different numerical methods for predicting the formation of the side ledge in an aluminum electrolysis cell, Light Met., 2009, pp. 563–568.
Severo, D. and Gusberti, V., A modeling approach to estimate bath and metal heat transfer coefficient, Light Met., 2009, pp. 557–562.
Poncsak, S., Kiss, L., Belley, A., Guerard, S., and Bilodeau, J.-F., Study of the structure and thermophysical properties of the side ledge in Hall-Heroult cells operating with modified bath composition, Light Met., 2015, pp. 655–660.
Sitnikov, A.V., Ershov, V.A., and Sysoev, I.A., Ways of measure of the working space in the production of aluminum in Nauchnye tendentsii: voprosy tochnykh i tekhnicheskikh nauk: Cbornik nauchnykh trudov po materialam VII mezhdunarodnoi nauchnoi konferentsii (Scientific Trends: Problems of Exact and Technical Sciences: Scientific Works of VII Int. Sci. Conf.), Samara, 2017, pp. 43–47.
Sitnikov, A.V., Ershov, V.A., and Sysoev, I.A., Laboratornye ispytaniya maketa dlya izmereniya formy rabochego prostranstva. in International Innovation Research: Sbornik statei XII Mezhdunarodnoi nauchno-prakticheskoi konferentsii (International Innovation Research: Collected Articles of XII Int. Sci.-Pract. Conf.), Pensa: MTsNS “Nauka i Prosveshchenie”, 2018, pp. 101–104.
Jianfei, Z., Dupuis, M., Feiya, Y., Xiaobing, Y., and Jun, H., Depth analysis and potentiality exploitation on energy-saving and consumption-reduction of aluminum reduction pot, Light Met., 2012, pp. 601–606.
Solheim, A. and Thonstad, J., Model experiments of heat transfer coefficients between bath and side ledge in aluminium cells, Metals, 1984, vol. 36, pp. 51–55.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by N. Korovin
About this article
Cite this article
Ivanova, A.M., Arkhipov, P.A., Rudenko, A.V. et al. Formation of Side Ledge and Bottom Ledge in an Aluminum Electrolyzer. Russ. J. Non-ferrous Metals 60, 624–631 (2019). https://doi.org/10.3103/S1067821219060075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821219060075