Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease
- 1Department of Horticulture and Landscape Architecture, Purdue University, West Lafayette, IN, United States
- 2Sustainable Agricultural Systems Laboratory, Henry A. Wallace Beltsville Agricultural Research Center, Agricultural Research Service (ARS-USDA), Beltsville, MD, United States
Biogenic amines—polyamines (PAs), particularly putrescine, spermidine and spermine are ubiquitous in all living cells. Their indispensable roles in many biochemical and physiological processes are becoming commonly known, including promoters of plant life and differential roles in human health and disease. PAs positively impact cellular functions in plants—exemplified by increasing longevity, reviving physiological memory, enhancing carbon and nitrogen resource allocation/signaling, as well as in plant development and responses to extreme environments. Thus, one or more PAs are commonly found in genomic and metabolomics studies using plants, particulary during different abiotic stresses. In humans, a general decline in PA levels with aging occurs parallel with some human health disorders. Also, high PA dose is detrimental to patients suffering from cancer, aging, innate immunity and cognitive impairment during Alzheimer and Parkinson diseases. A dichotomy exists in that while PAs may increase longevity and reduce some age-associated cardiovascular diseases, in disease conditions involving higher cellular proliferation, their intake has negative consequences. Thus, it is essential that PA levels be rigorously quantified in edible plant sources as well as in dietary meats. Such a database can be a guide for medical experts in order to recommend which foods/meats a patient may consume and which ones to avoid. Accordingly, designing both high and low polyamine diets for human consumption are in vogue, particularly in medical conditions where PA intake may be detrimental, for instance, cancer patients. In this review, literature data has been collated for the levels of the three main PAs, putrescine, spermidine and spermine, in different edible sources—vegetables, fruits, cereals, nuts, meat, sea food, cheese, milk, and eggs. Based on our analysis of vast literature, the effects of PAs in human/animal health fall into two broad, Yang and Yin, categories: beneficial for the physiological processes in healthy cells and detrimental under pathological conditions.
Introduction
Polyamines (PAs) are aliphatic polycations ubiquitously present in all tissues and all cell types examined in animals and plants. PAs have specific and diverse roles in multiple cellular processes, including apoptosis, cell division and differentiation, cell proliferation, DNA and protein synthesis, gene expression, homeostasis, signal transduction (Cohen, 1998; Kaur-Sawhney et al., 2003; Kusano et al., 2008; Anwar et al., 2015; Pegg, 2016). Although, interest in the biology and mechanism of action of PAs was sporadic since the initial discovery of spermine (Spm) phosphate crystals in human semen (~1678 AD), it is now known that PAs and their analogs have functional implications in plant life as well as in various aspects of human/animal health and disease. For instance, their role(s) are recognized in gastroenterology (Pfeffer et al., 2001), programmed cell death (PCD) (Pfeffer et al., 2001; Pignatti et al., 2004; Moschou and Roubelakis-Angelakis, 2014; Cai et al., 2015); parasitology (Bacchi and Yarlett, 2002), cerebral stroke and other disorders (Tomitori et al., 2005), oxidative stress (Tomitori et al., 2005; Agostinelli et al., 2006; Cona et al., 2006), oncology (Casero and Marton, 2007), and obesity (Jell et al., 2007). Likewise, in plants PAs feature in a large range of processes including plant growth and development and, in particular, stress/senescence responses (Cohen, 1998; Kaur-Sawhney et al., 2003; Capell et al., 2004; Tang and Newton, 2005; Handa and Mattoo, 2010; Bitrián et al., 2012; Pathak et al., 2014; Tiburcio et al., 2014; Anwar et al., 2015; Liu et al., 2015; Gupta et al., 2016; Sobieszczuk-Nowicka, 2017). These findings have intensified research on elucidating function(s) of PAs in pharmacology and medicine (Bachrach and Wang, 2002; Janne et al., 2004; Agostinelli et al., 2006; Guerra and Rubin, 2016; Pegg, 2016), modulating pre- and postharvest biology (Paschalidis and Roubelakis-Angelakis, 2005; Uemura et al., 2005; Nambeesan et al., 2010, 2012) and abiotic and biotic stresses in plants (Yamaguchi et al., 2006; Prabhavathi and Rajam, 2007; He et al., 2008; Wen et al., 2008; Alcazar et al., 2010a,b; Mattoo et al., 2015).
Main PAs in mammalian cells and plants are putrescine (Put), spermidine (Spd) and spermine (Spm), with thermo-Spm (T-Spm) discovered in plants to be important against abiotic stress. Other than these PAs, flowering plants also synthesize cadaverine, 1,3-diaminopropane, and other modified forms. Other amines include cadaverine, homoSpd, norSpd, homoSpm, norSpm, thermoSpm, aminopropyl homoSpd, and methyl Spd, which are less predominant (Grimes et al., 1986; Tiburcio et al., 1993; Hamana et al., 1998; Martin-Tanguy, 2001). The presence of branched and long PAs generally present in legumes and graminae seeds are thought to protect the seed from water deficit, while di-, tri-, and tetra-PAs in aquatic plants may regulate osmotic stress during submergence in water (Hamana et al., 1998).
Biochemical roles of PAs have been recognized in several processes, including the synthesis, maintenance of structure, stability and functions of proteins and nucleic acids (Park, 2006). Examples include, but are not limited to, the posttranslational maturation of Eukaryotic Translation Initiation Factor 5A (eIF5A) by the conversion of Lys to Spd-derived hypusine, which is essential for translation of mRNAs of several proteins containing polyproline tracts or triplets of PPX (X being Gly, Trp, Asp, or Asn). These proteins are essential for viability playing roles in DNA binding, transcription, RNA splicing and turnover, cell signaling, actin/cytoskeleton-associated functions, nucleocytoplasmic transport and apoptosis (Nishimura et al., 2012; Caraglia et al., 2013; Mandal et al., 2014; Sievert et al., 2014; Mathews and Hershey, 2015; Pällmann et al., 2015). PA binding to the inwardly rectifying K+ (Kir) channels facilitates movement of K+ into the cell and affects resting membrane potential, electrolyte balance, and cardiac and electrical activity and controlling the electrical excitation in many types of cells (Hibino et al., 2010; Baronas and Kurata, 2014). Spd binding to NMDAr, AMPAr and kainite influence their function including in animal/human memory (Williams et al., 1989; Bowie and Mayer, 1995; Williams, 1997). PAs also regulate C, transient receptor potential cation (TRPC) channels and connexins to regulate the gastrointestinal smooth muscle excitability and contractility (Pegg, 2016). Genomic and metabolomic studies have identified gene medleys and metabolic pathways regulated by PAs, impacting cellular metabolism and involving processes in subcellular compartments from nucleus to mitochondria and cytoplasm. In plants as well, PAs abound in cell organelles, including nucleus, chloroplast, mitochondria and chromoplasts in different forms—as free amines, conjugated to small molecules, and bound to larger macromolecules (Martin-Tanguy, 2001).
Metabolite profiling has identified Spd and Spm as regulators of nitrogen (N) and carbon (C) metabolism, causing accumulation of other N forms such as Glu, Gln and Asn. The sensing of PAs and their signaling responses are common responses of plant roots, leaves and trees to exogenous N (Rennenberg et al., 1998; Foyer and Noctor, 2002; Bauer et al., 2004; Mattoo et al., 2006; Mattoo and Handa, 2008). The N assimilation and C metabolism in different plant organs seems conserved, contributing to the totipotent nature of plant cells. Regulation of N and C use pathways also became prominent through studies with transgenic poplar cell culture lines that accumulated high Put levels in comparison to their low Put-producing line. Higher Put levels were associated with increased flux of glutamate into ornithine, while this also caused concurrent enhancement in glutamate production involving more N and C assimilation (Page et al., 2016).
Also, upon reaching a threshold in cellular PAs, cells down-regulate assimilation of nitrate-derived N (Provan et al., 2000; Shen and Huber, 2006), supporting the notion that PAs may also be signals of nitrogen-replete conditions. One way of achieving this regulation is by stimulating specific protein–protein interactions (Aitken, 1996; Provan et al., 2000; Bridges and Moorhead, 2004; Shen and Huber, 2006; Garufi et al., 2007). A positive role of Put in mitigating a destructive disease in the form of mango malformation further suggests that PAs regulate specific developmental processes in plants (Singh et al., 2014). Growth and life span in eukaryotes is regulated by a major nutrition and energy sensor called Target of Rapamycin (TOR). Among many functions of TOR include regulation of anabolic pathways involving polysome integrity, translation of mRNA and ribosome biogenesis (Proud, 2004; Tavernarakis, 2008; Ren et al., 2012), functions similar to PAs. It is interesting that PA biosynthesis in plants seems connected to TOR function in the plant model Arabidopsis (Ren et al., 2012). In this context, PAs have been implicated in TOR signaling and prostate cancer (Zabala-Letona et al., 2017). Together, these findings demonstrate that some functions of PAs are conserved, providing parallels between plants and humans.
Biosynthesis of PAs
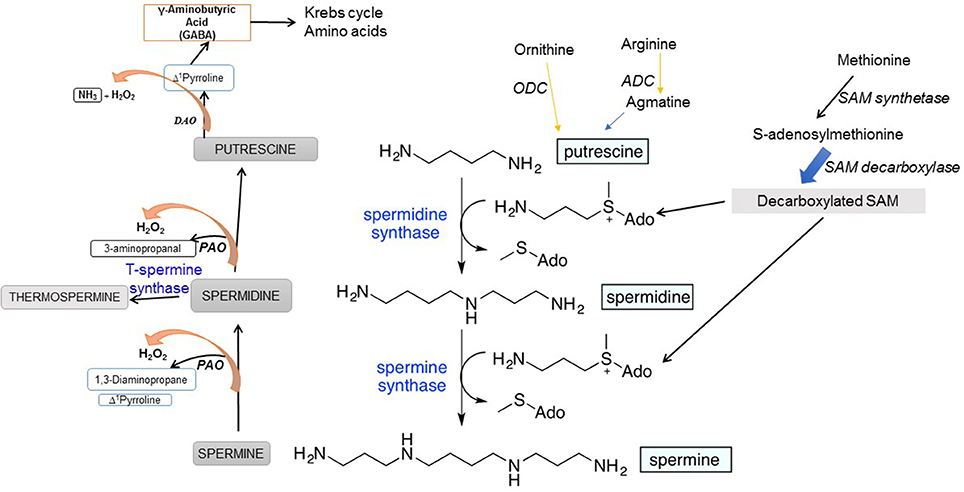
PA biosynthesis initiates from two amino acids, arginine (Arg) and ornithine (Orn). In animals, Arg is first converted by mitochondrial arginase to Orn, which is then decarboxylated by ODC to synthesize Put. Plants use an additional pathway involving arginine (Arg) decarboxylation by Arg decarboxylase (ADC) to produce Put (Fig. 1). Decarboxylated arginine—agmatine—is synthesized by plants and by many bacteria, including the intestinal flora but not in mammals (Coleman et al., 2004; Pegg, 2016) where its source is either food ingested or the intestinal microflora. Decarboxylation of S-adenosylmethionine (SAM), catalyzed by SAM decarboxylase (SAMDC), yields aminopropyl group that acts as a substrate together with Put for Spd synthase and generates Spd followed by the incorporation of another aminopropyl group (also from decarboxylated SAM) catalyzed by Spm synthase to form Spm (Figure 1). Spd is also converted to a Spm isomer, thermospermine (T-Spm), a reaction catalyzed by T-Spm synthase (Figure 1). T-Spm has been shown to be required for normal growth and development, promoting stem elongation and suppresses auxin-induced xylem differentiation in Arabidopsis (Takano et al., 2012; Yoshimoto et al., 2016 and references therein). ODC in mammals requires pyridoxal phosphate (PLP) as a cofactor (Lee et al., 2007) while other SAMDCs have covalently bound pyruvate for catalysis (Tolbert et al., 2001). In addition to the difference between plants and animals in the initial reactions catalyzing the formation of Put, the latter also synthesize ODC antizyme that inhibits ODC enzymatic activity and regulates the Put homeostasis. The antizyme inhibits ODC and also targets the latter to the 26S proteasome for degradation—a process in animal cells to maintain cellular homeostasis of ODC activity (Wallace et al., 2003; Janne et al., 2004; Igarashi and Kashiwagi, 2010; Perez-Leal and Merali, 2011).
Polyamine biosynthetic pathway and genes encoding the enzymes involved have been identified and characterized in both prokaryotes and eukaryotes (Coleman et al., 2004; Kusano et al., 2007; Pegg, 2016). In Lathyrus sativus, an additional/alternate pathway of Spd synthesis exists which involves formation of a Schiff base between aspartic 4-semialdehyde with Put to yield carboxy-Spd as an intermediate that undergoes a pyridoxal phosphate-dependent enzymatic decarboxylation to give rise to Spd (Srivenugopal and Adiga, 1980). Such a pathway is known to be present in prokaryotes and was demonstrated and characterized in Vibrio cholera (Lee et al., 2009).
Comparison of the Content of PAs in Plants and Animals
The consumer demand for bountiful nutritious produce has intensified since the recognition that biomolecules such as antioxidants (including phenolic compounds), vision-improving carotenoid β-carotene, and anti-cancerous carotenoid lycopene have the potential to enhance quality of life in humans and animals. Thus, due to their anti-oxidant and anti-inflammatory attributes, PAs as well have been implicated in beneficial effects on human health (Lagishetty and Naik, 2008). However, PA levels generally decline with aging in most organisms, from bacteria to mammals, which may play a significant role in aging and associated diseases. The aging-associated decline in PA levels is reversed by dietary PA supplementation of a healthy population (Soda et al., 2009b). Studies on implications of food-based PAs for animal growth and health found them to be essential for normal metabolism in rat tissues and organs that had been stimulated to grow by metabolic signaling (Bardócz et al., 1993). Biological implication was studied using human population, which proposed that both endogenous and dietary PAs could be useful for post-operation patients during wound healing, and for the growth and development of neonate's digestive system (Kalac and Krausova, 2005).
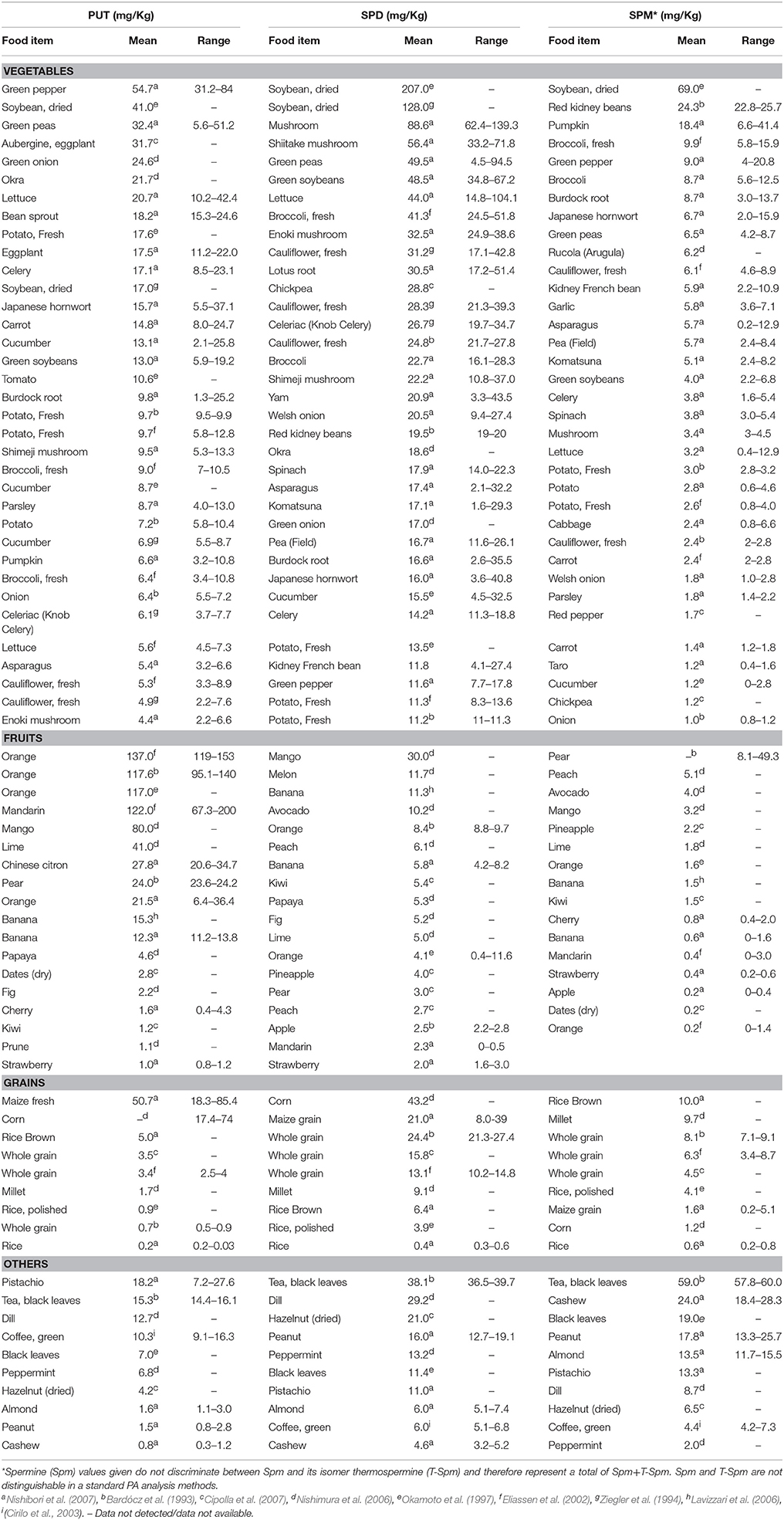
Fruits and their juices, sauerkraut, ketchup, frozen green peas and fermented soybean products contain high levels, above 40 mg kg−1, while legumes (mainly soybean), pear, cauliflower and broccoli commonly accumulate high Spd content than Spm, usually above 30 mg kg−1 FW (Kalac and Krausova, 2005). Based on the published literature, low levels of Put are typical for well-treated foods of animal origin in contrast to foods of plant origin (Kalac and Krausova, 2005). Tables 1, 2 summarize the distribution of different PAs in selected plants and animals found in the literature. The reported quantities are given as mg.kg−1 in their descending order (Nishibori et al., 2007; Ali et al., 2011).
PAs in Plant Origin Food
PAs in plant-based diet have been mostly studied in USA, Japan, UK, France, Germany, Spain, Sweden, and Norway. The levels of Put, Spd, and Spm in vegetables, fruits, grains and other plant-based foods are summarized in Table 1. Vegetables rich in Put include green pepper, dried soybean, green peas, eggplant and green onion. High amounts of Put, in the range of 31.2–84 mg kg−1 FW with a mean value of 54.7 mg kg−1 FW, were found in Japanese fresh green pepper (Nishibori et al., 2007). Spd- and Spm-rich vegetables are mushrooms, broccoli, lettuce and pumpkin, along with beans. Dried soybean (Put 41, Spd 207, and Spm 69.0, mg kg−1 FW) and green peas (Put 32.4, Spd 49.5, and Spm 6.5, mg kg−1 DW) are among the top 10 vegetables rich in all the three PAs (Okamoto et al., 1997; Nishibori et al., 2007). Overall, beans seem to have higher levels of Spd and Spm while many vegetables are higher in Put and Spd (Table 1). Orange, mango, and banana feature among the top ten fruits highly-enriched in PAs, with Put being the main PA followed by Spd (Bardócz et al., 1993; Okamoto et al., 1997; Eliassen et al., 2002; Nishimura et al., 2006; Table 1). Fruits other than these, such as lime (41.0 mg kg−1 FW Put), pear (24.0 mg kg−1 FW Put) and melon (11.7 mg kg−1 FW Spd) are also good sources of PAs (Bardócz et al., 1993; Nishimura et al., 2006).
Cereals are among the most staple source of carbohydrates and fiber for human diet. Notably, maize (corn), brown rice and whole grain wheat are richer in Spd (Bardócz et al., 1993; Okamoto et al., 1997; Eliassen et al., 2002; Nishimura et al., 2006; Nishibori et al., 2007), with the following decreasing order: Japanese corn (43.0 mg kg−1 FW) > whole grain wheat (13.1–21.0 mg kg−1 FW) > millet (9.1 mg kg−1 FW) > brown rice (6.4 mg/kg −1FW). Interestingly, all these grains contain more Spm than Put. In addition to vegetables, fruits and grains, nuts are also a good source of PAs. Pistachio and almonds contain high levels of all three major PAs (Nishibori et al., 2007) whereas hazelnut (Cipolla et al., 2007) and cashew (Nishibori et al., 2007) contain higher amounts of Spd (21 mg kg−1 FW) and Spm (24 mg kg−1 FW), respectively.
In the context of common beverages, tea and coffee are the two most favorite beverages consumed by humans. Black tea leaves are rich in Spm (59 mg kg−1) followed by Spd (38.1 mg kg−1) and Put (15.3 mg kg−1) while the pattern of PAs content in green coffee was found to be in the reverse order, with Put at 10.3 mg kg−1 significantly higher than Spd (6.0 mg kg−1) and Spm (4.4 mg kg−1), respectively (Bardócz et al., 1993; Cirilo et al., 2003).
In certain diets, spices are an integral part where they not only provide flavor but also are thought to help fight infection, boost immune system, reduce inflammation, and prevent cancer and heart diseases. Among such spices, peppermint contains 2 mg kg−1 Spm and dill contains 29.2 mg kg−1 Spd (Nishimura et al., 2006).
PAs in Animal Origin Food
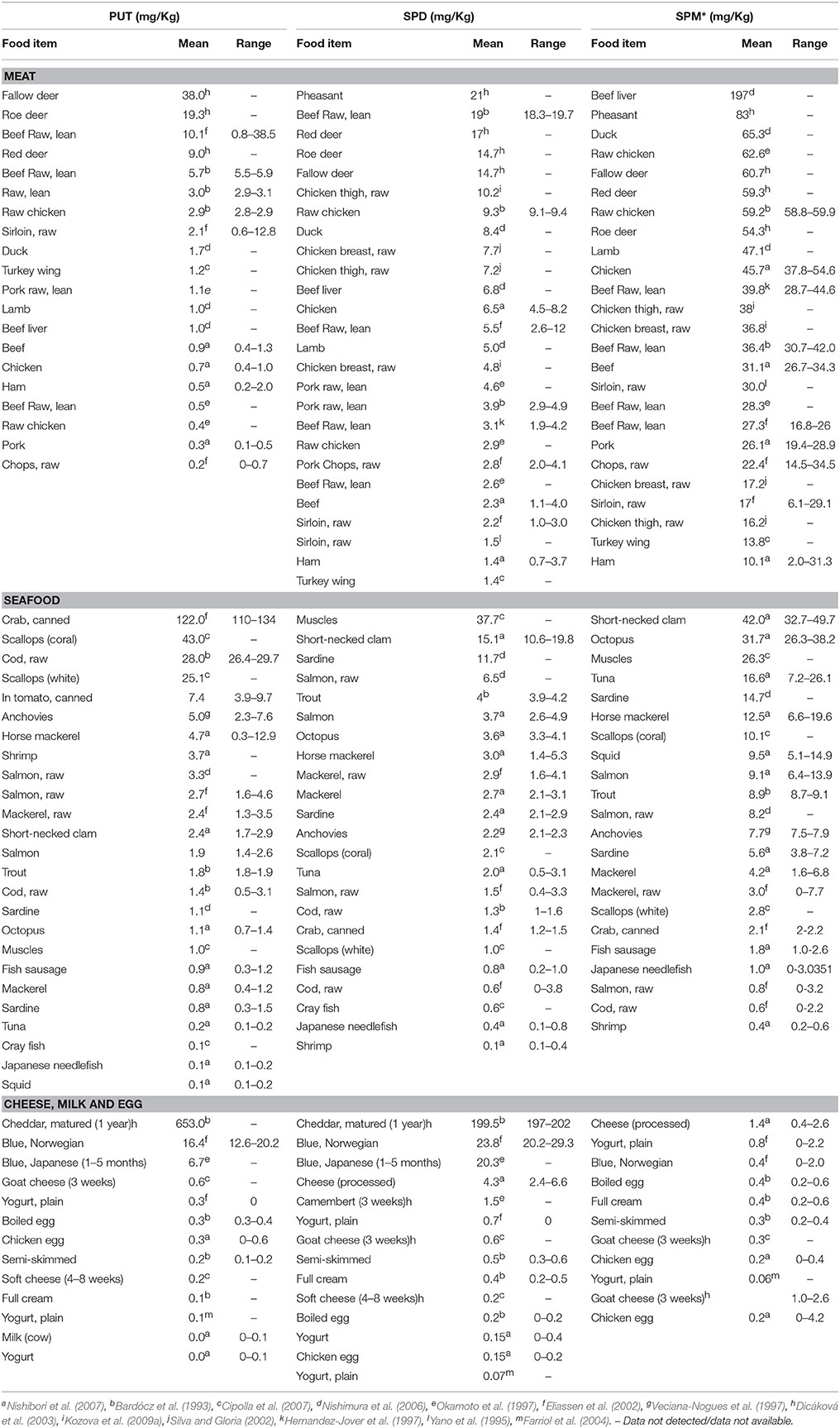
PA content in meats, seafood and dairy products is summarized in Table 2. Many studies have indicated that Put levels in animal meat are generally lower (less than 10 mg kg−1) as compared to Spm (more than 25 mg kg−1) (Bardócz et al., 1993; Yano et al., 1995; Hernandez-Jover et al., 1997; Okamoto et al., 1997; Veciana-Nogues et al., 1997; Eliassen et al., 2002; Silva and Gloria, 2002; Dicáková et al., 2003; Farriol et al., 2004; Nishimura et al., 2006; Cipolla et al., 2007; Nishibori et al., 2007; Kozova et al., 2009b). Pheasant, duck, deer, chicken, lamb and pork contain higher levels of Spm compared to Spd and Put, with beef liver containing the highest levels, 197 mg kg−1 FW, of Spm (Nishimura et al., 2006). However, fallow deer contains higher levels of Put (38 mg kg−1 FW) than Spd (14.7 mg kg−1 FW) (Dicáková et al., 2003). Among the seafood—canned crab, coral scallops, raw cod, and shrimp do not follow any typical pattern, i.e., high Spm and low Put, except they are richer in Put (Bardócz et al., 1993; Eliassen et al., 2002; Cipolla et al., 2007). On the other hand, short-necked clam, muscles, octopus, salmon and sardine are rich sources of Spm and Spd while canned crab (122 mg.kg−1), scallops (43 mg.kg−1), raw cod (28 mg.kg−1), and shrimp (3.7 mg.kg−1) are more enriched in Put (Ali et al., 2011). Spm levels in meat and meat products of warm-blooded animals are generally higher, ranging from 20 and 60 mg kg−1 whereas fish contain < 10 mg kg−1 (Kalac and Krausova, 2005). It is apparent that high concentrations of Spd and Spm is typical of foods from animal origin as compared to the plant products (Kalac and Krausova, 2005).
Also, extremely high levels of Put and Spm are present in matured cheddar cheese (Dicáková et al., 2003), Blue Norwegian cheese (Eliassen et al., 2002) and Blue Japanese cheese (Okamoto et al., 1997). In comparison, PA concentration is low in milk, yogurt, and eggs (Bardócz et al., 1993; Farriol et al., 2004; Nishimura et al., 2006).
Storage and Processing of Tissues/Cells Affect PA Content
Storage of leafy vegetables—Chinese cabbage, endive, iceberg lettuce and radicchio, at 5°C for more than 5 days increased their Put levels from 3- to 8-fold without any significant change in Spd and Spm levels (Simon-Sarkadi et al., 1994). Significant reduction in the levels of Spd with a complete loss of Put and Spm was achieved after green coffee was roasted (Cirilo et al., 2003). On the other hand, the fermentation of soy-based products results in elevated levels of PAs (Okamoto et al., 1997). Irradiation of Korean soybean paste before fermentation decreased the levels of Put and Spd but not Spm (Kim et al., 2003). Similarly, cooking of vegetables—broccoli, cauliflower, savoy, and asparagus causes significant losses in PA levels due to leaching in water (Ziegler et al., 1994).
A very elaborate information about free and conjugated PAs in fermented foods consumed by Japanese is also available (Okamoto et al., 1997). In short, the typical fermented food items, miso and soy sauce, were found to be high in Put and Spd but almost deficient in Spm. The differences between PAs profiles of soy sauces and soybeans confirmed that PAs are produced upon fermentation, due to microbial decarboxylation. In comparison to soy sauce and miso, natto and tempe—relatively simple forms of fermented soybean—possess lesser amounts of PAs than soybean due to the degradation of some PAs during fermentation.
PAs in beef, pork and lamb stored at 5°C have also been analyzed (Edwards et al., 1983). Put levels increased over 13 days of storage. However, when vacuum-packed beef sirloin was stored up to 39 days at 0, 5, or 10°C, Spd and Spm contents did not change (Yano et al., 1995). In summary, storage and processing—be it fermentation, roasting, ripening or cooking, results in alterations in the levels and accumulation patterns of specific PAs.
Homeostatic Regulation of PAs and H2O2 Evolution in Plants
Cellular PA homeostasis is achieved via interconversion of PAs, process catalyzed by PA amine oxidase (PAO) and diamine oxidase (DAO) enzymes. Such enzymatic reactions lead to PA catabolism and also the production of H2O2 as one of the products (Figure 1). PAO/DAO catalysis that generates H2O2 from PAs adds another dimension to the function of PAs in living cells, particularly because of the potential of H2O2 both as a secondary messenger and a signaling molecule (Cona et al., 2006; Tavladoraki et al., 2012). In the back-conversion reactions, Spm is oxidized to Spd, and Spd can also be oxidized to Put (Figure 1).
PAOs catalyze flavine adenine nucleotide (FAD)-dependent oxidation of Spd and Spm and/or their acetylated forms while the preferred substrate for copper amine oxidases (CuAOs) is Put (Cona et al., 2006; Tavladoraki et al., 2012). Five PAO genes (AtPAOs) and ten CuAO genes (AtCuAOs) are present in a model plant Arabidopsis (Fincato et al., 2011; Planas-Portell et al., 2013; Ahou et al., 2014; Kim et al., 2014). The functions of PAOs include light-induced inhibition of the mesocotyl growth (Cona et al., 2003), cell death (Tisi et al., 2011a), wound-healing (Angelini et al., 2008), salt stress (Moschou et al., 2008), JA-induced root xylem differentiation (Ghuge et al., 2015), and pathogen attack (Moschou et al., 2009) to name a few. PA-derived H2O2 triggers signal transduction pathways causing defense gene expression, stress tolerance, or cell death (Moschou et al., 2008; Tisi et al., 2011b). ROS signaling involving oxidative PA catabolism is situational, for instance, during stress where plant growth is under stress (Ghuge et al., 2015). The apoplastic copper amine oxidase AtAO1 mediates jasmonic acid-induced protoxylem differentiation in Arabidopsis roots. Another metabolic salvage pathway associated with DAO-mediated oxidation of Put which generates pyrroline which is metabolized to γ-aminobutyric acid (GABA) and the latter feeds into Krebs cycle and N metabolism (Figure 1).
PAs and Abiotic Stress in Plants
Progress in elucidating role(s) of PAs in plants was catalyzed when gain-of-function and loss-of-function genetic plants with modified levels and/or the type of PA were developed and analyzed (Noh and Minocha, 1994; Kumar et al., 1996; Mehta et al., 2002; Capell et al., 2004; Nambeesan et al., 2010, 2012; Majumdar et al., 2013). These transgenic approaches brought to light PA roles in plants as follows: extending fruit life on planta, delayed seasonal whole plant senescence, enhanced anabolic and N-C cellular interactions Mehta et al., 2002; Mattoo et al., 2006; Nambeesan et al., 2010), accumulation of anti-cancer molecules such as lycopene (Mehta et al., 2002), role of Orn in regulating PA homeostasis and Glu levels (Majumdar et al., 2013); and tolerance to abiotic stresses including drought and salinity (Capell et al., 2004; Cona et al., 2006; Kusano et al., 2007; Alcazar et al., 2010b; Shukla and Mattoo, 2013; Minocha et al., 2014; Mattoo et al., 2015; Pal et al., 2015). Thus, transgenic tobacco, rice, and tomato plants engineered to accumulate PAs by overexpression of SAM decarboxylase showed tolerance against salt, osmotic, and heat stresses (Wi et al., 2006; Alcazar et al., 2010a), and engineered Spd overexpression in Arabidopsis, pear and potato plants enabled these transgenic plants to be tolerant against drought, salt and oxidative stresses (Kasukabe et al., 2004, 2006; He et al., 2008; Wen et al., 2008; Alcazar et al., 2010a). In contrast, and as a confirmation of the above findings, PA pathway-deficient Arabidopsis mutants were more sensitive to salt stress (Watson et al., 1998; Kasinathan and Wingler, 2004; Urano et al., 2004; Yamaguchi et al., 2006).
PAs and Human Health
Phenotypes Associated with Polyamine-Deficient Mutants
Functions of PAs in mammals has been recently reviewed (Pegg, 2016). Since polyamines play significant roles in various physiological processes, mutations resulting in the loss of either Put, Spd, or Spm have been linked to various phenotypes in many organisms including humans. Spd is the precursor of hypusine which is required for the post-translational modification of the elongation factor eIF5A and its deficiency thereof affects protein function (Pegg, 2016). The function of Spm emerged with the characterization of a male offspring of a mice mutant, designated as Gyro, gene sumbol Gy (Lyon et al., 1986). This mutant was deficient in the synthesis of Spm, due to a deletion in the Spm synthase (SpmSyn) gene along with a part of the adjacent Phex gene. This mutation led to several phenotypes, including hypophosphatemia with rickets/osteomalacia and circling behavior, reduced size, sterility, deafness, neurological abnormalities, and short life span due to sudden death (Lyon et al., 1986; Lorenz et al., 1998). The hypophosphatemia and reduced size in Gy mice were associated with the partial loss of the Phex gene, while other phenotypes were attributed to the reduced levels of Spm (Meyer et al., 1998). Essential role of Spm in humans became apparent after molecular characterization of an X-linked recessive condition in males associated with intellectual disability disorder known as Snyder-Robinson syndrome (SRS) (Albert et al., 2013). The SRS is also associated with osteoporosis, dysmorphic faces, speech and gait abnormalities, seizures, muscle hypoplasia, and kyphoscoliosis (Albert et al., 2013). The SRS syndrome was shown to result from the inaccurate splicing of SpmSyn mRNA, causing production of inactive truncated protein and low levels Spm (Cason et al., 2003).
Aging
Cellular levels of Spd and Spm decrease during aging of many organisms (Nishimura et al., 2006; Minois et al., 2011; Zwighaft et al., 2015). This decline in PA levels plays significant role in the aging process of organisms, from bacteria, fungi, plant, to mammals (Eisenberg et al., 2009; Nambeesan et al., 2010). Spd levels in human population are variable, it is higher in those under 50 years of age, lower in those within 60–80 years of age, while those who were 90 years or older had Spd levels similar to the younger population (<50 years). These results were interpreted to suggest that higher Spd levels play a role in human longevity (Pucciarelli et al., 2012; Minois, 2014). Studies using dietary supplementation with exogenous Spd, which ameliorated many of the deleterious physiological effects of aging in mice, fruit fly and C. elegance and led to 15–40% increase in their life span, have supported the contention that Spd is good for healthy subjects (Eisenberg et al., 2009; Soda et al., 2009a,b). Similarly, addition of Spd to aging yeast cultures resulted in about 4-times increase in their clonogenic survival while similar treatment of human peripheral blood mononuclear cells increased their viability from 15 to 50% in a 12-days old culture cells (Eisenberg et al., 2009). Also, incidence of age-related kidney glomerular atrophy and DNA methylation were found to decrease with high PA diet fed to mice (Soda et al., 2009a,b). Exogenous Spd also reduced the age-associated overproduction of reactive oxygen and oxidative damage in yeast, fruit fly and mice, and imparted heat tolerance to yeast and oxidative stress tolerance to fruit fly (Eisenberg et al., 2009; Soda et al., 2009a,b). In plants, engineering high endogenous Spd and Spm levels prolonged the phase of tomato fruit ripening, the terminal developmental stage in the ontogeny of fruit, and increased fruit shelf life up to 40% compared to low-PA fruit (Mehta et al., 2002; Nambeesan et al., 2010). Enhanced autophagy by higher PA levels has been suggested to be the primary mechanism by which PAs affect the aging process (Eisenberg et al., 2009).
Many organisms, including Drosophila, mouse and human, show age-dependent impairment in memory that is associated with a decrease in PAs, especially Put and Spd. Although the molecular basis of such impairment is not known, PAs have been shown to play essential role in both aging and age-associated memory loss. Spd feeding to aging fruit flies restored juvenile PA levels concomitant with recovery of the age-induced memory (Gupta et al., 2013). In addition to the restoration of memory loss, the Spd-fed fruit flies were found to undergo higher levels of autophagy. Both of these processes remained impaired in the fruit flies that carried a mutation in the autophagy process. The ectopic expression of ODC gene under neuron-specific appl-Gal4 promoter protected fruit flies against age-induced memory loss. Taken together, these results indicate a role of Spd-induced autophagy in resorting the age- associated memory loss (Gupta et al., 2013). In addition, these results provide evidence for the diet-based PA acquisition for maintaining memory during aging.
Effects of higher PAs in general and Spd in particular on aging have been attributed to several biochemical mechanisms including increased autophagy, lipid metabolism, cell growth and death processes. Autophagy plays a positive role in slowing the aging process by removing the damaged proteins and organelles from cells (Yamaguchi and Otsu, 2012). PAs, particularly Spd, were shown to increase autophagy in many organisms, implicating them in enhancing their life-span (Eisenberg et al., 2009; Minois et al., 2014). Spd was also found to reduce necrosis in yeast by inhibiting aging, one of the factors considered to regulate this process (Eisenberg et al., 2009). Spd inhibits histone acetylases, thereby increasing longevity by enhancing cytoprotection, a mechanism similar to that suggested for an antioxidant resveratrol that activates histone deacetylase Sirtuin 1 to confer longevity (Morselli et al., 2010). However, it remains to be determined if the same histones are targets for both Spd and resveratrol.
Memory
Preexisting Memory
Seminal neurochemical and neuro-physiological studies have provided a wealth of information on the role of PAs in the central nervous system (Seiler and Schmidt-Glenewinkel, 1975; Ransom and Stec, 1988; Sacaan and Johnson, 1990; Williams et al., 1994; Shaw et al., 2001). The N-methyl-D-aspartate (NMDA)-glutamate receptor plays a role in spatial learning which became apparent when infusion of its antagonists selectively impaired learning (Morris et al., 1986). Dizocilpine (MK-801) is an uncompetitive antagonist of NMDAr and is reported to impair learning. By interacting with NMDAr, Spd potentiates the effect of MK-801 on learning impairment (Shimada et al., 1995). Spd also attenuated working memory acquisition deficit induced by intrahippocampal MK-801, scopolamine (muscarinic antagonist) and AIDA (a metabotropic glutamate receptor class I antagonist) suggesting that Spd affects memory by binding to NMDAr (Kishi et al., 1998a,b). Earlier it had been shown that brain Spd, but not Spm, levels increased during the social-isolation associated aggressive behavior and decreased upon transferring the animal to its colony (Tadano et al., 2004). Other studies have reported that higher levels of PAs, specifically Spm, were toxic to sedation, hypothermia, anorexia, adipsia, seizures and focal encephalomalacic lesions (Rosenthal et al., 1952; Anderson et al., 1975). An intracerebroventricular treatment with 125 nmol Spm potentiated the deleterious effects of diazepam and abolished previously-learned platform position (Conway, 1998).
Trained Learning and Memory
Many studies have focused on the effects of Spd on shock-motivated learning and memory including during stepdown inhibitory and passive avoidance tasks, fear conditioning acquisition and consolidation. Intraperitoneal, intracerebroventricular, intrahippocampal, or intra-amygdala delivery of Spd (0.02–20 nmol) improved early consolidation during stepdown and inhibitory avoidance tasks (Guerra and Rubin, 2016). Arcaine (a potent antagonist for the PA binding site on the NMDAr) at 0.002–0.2 nmol had either no effect or prevented Spd-associated consolidation during stepdown and inhibitory avoidance tasks. Spd (0.02–2 nmol) generally improved fear conditioning acquisition and consolidation whereas arcaine (0.0002–0.02 nmol or 10–30 mg/kg) treatment impaired this acquisition (Rubin et al., 2004; Camera et al., 2007; Da Rosa et al., 2012; Signor et al., 2014; Guerra and Rubin, 2016 and references therein). PAs also modify reconsolidation and extinction of fear conditioning and step-down avoidance, but the observed effects evaluated by different investigators were variable. The intraahippocampal treatment with 2 nmol Spd facilitated fear extinction in one investigation (Gomes et al., 2010) but not in another investigation at 10 nmol Spd (Bonini et al., 2011). Yet in another investigation, the intraahippocampal Spd treatment at 0.02–0.2 nmol improved memory consolidation. PAs generally potentiated the deleterious effects of diazepam MK-801 as determined by Morris-water maze, object recognition and place conditioning. However, the intraperitoneal injection of 5.0–20 mg/kg Spd improved social memory. Spm at 10–125 nmol potentiated detrimental effects of diazepam and tended to impair objective recognition memory (see Guerra and Rubin, 2016 and references herein).
Cancer
PAs are well known to be involved in cell division, proliferation, and pro-growth metabolism that can cause proliferation in normal cells (Slocum and Flores, 1991; Cohen, 1998; Cassol and Mattoo, 2003; Kaur-Sawhney et al., 2003; Janne et al., 2004). Although there is no direct evidence that PAs as such can initiate cancer, caution has been expressed to their deployment/ingestion in situations where the cells suffer from uncontrolled growth and disease, for example cancer. Interest in PAs as promoters of cellular proliferation and growth developed after reports on the presence of ODC activity in regenerating rat liver, chick embryo, and various tumors (Russell and Snyder, 1968) and increase in PAs levels in patients with familial adenomatous polyposis (FAP), a genetic form of colon cancer (Giardiello et al., 1997). The observation that cancerous cells and liver of tumor-bearing mice (Andersson and Heby, 1972), and cancerous colon, breast and skin (Upp et al., 1988; Manni et al., 1995; Gilmour, 2007) accumulate elevated levels of PAs suggested their possible association with cancerous cells (Wallace and Caslake, 2001). These studies added further caution about feeding PAs or diets rich in them to patients with growth abnormalities. This led to a rationale put forth to use an inhibitor of ODC, difluoromethylornithine (DFMO), as a chemopreventive agent (Nowotarski et al., 2013). Thus, reduction of exogenous PAs from food and gut microbiome, or inhibition of PAs biosynthesis, are some strategies in vogue to test their usefulness during chemotherapy.
Several studies have been conducted where tumor-bearing animals were fed a Polyamine Reduced/Deficient Diet (PRD/PDD) or treated with DFMO and neomycin (for partial decontamination of the gastro-intestinal tract) to determine the effects of these treatments on remission/ reduction of tumor in these animals. The results from these investigations showed significant inhibition of tumor progression and spreading metastasis, as well as stimulation in anticancer immunity, without inducing deleterious secondary effects (Seiler et al., 1990; Chamaillard et al., 1997). The tumor-grafted animals fed only on PDD together with neomycin in the drinking water (i.e., without DFMO) also exhibited about 40% inhibition in tumor progression and metastasis spreading (Seiler et al., 1990). Neither neomycin nor DFMO was able to positively modify the malignant evolution. Thus, such positive data led to clinical trials with a PA-free oral nutritional supplement (ONS) combined with docetaxel fed to castrate-resistant prostate cancer (CRPC) patients, but yielded little side effects (Artignan et al., 2012). It has been suggested that PAs do not trigger cancer, but accelerate tumor growth (Kalač, 2014).
Innate Immunity
Several factors modulate innate immunity in animal models (Brubaker et al., 2015). These include structural components of bacteria, fungi, virus, and their metabolites, such as nucleic acids that activate germline-encoded pattern-recognition receptors (PRRs) to induce/enhance innate immunity (Medzhitov, 2009). Most PRRs are divided into 6 families designated as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), nucleotide binding domain, leucine-rich repeat (LRR)-containing (or NOD-like) receptors (NLRs), RIG-like receptors (RLRs), and AIM2-like receptors (ALRs). These families are further subdivided into membrane bound and free intracellular receptors (Brubaker et al., 2015). The PRRs act primarily as transcriptional regulators for the production of cytokines and interferons (IFN) - the proinflammatory chemical messages that initiate innate and adaptive immune responses. Some PRRs also initiate nontranscriptional responses, including phagocytosis, autophagy, cell death, and cytokine processing (Deretic et al., 2013; Lamkanfi and Dixit, 2014). Signal transduction pathways tightly control the transcriptional as well as nontranscriptional innate immune responses by PRR-mediated microbial detection (Palm and Medzhitov, 2009).
PAs modify the innate immune responses by activating TLRs that detect various microbes in the vicinity and activate innate immunity after infection (Medzhitov, 2001). The TLRs represent a family of single membrane-spanning non-catalytic receptor proteins capable of recognizing structurally conserved microbial molecules such as lipopolysaccharides (LPS) (Pirnes-Karhu et al., 2012). Interestingly, LPS are reported to induce expression of ODC in neurons and microglia across the mouse central nervous system (CNS) and increase transiently the transcription of genes encoding pro-inflammatory cytokines and TLR2 in microglial cells (Soulet and Rivest, 2003). Inhibition of ODC activity by its inhibitor DMFO ameliorated the LPS-associated increase in ODC and lowered Put levels in cells, resulting in increased survival rate of mice by abolishing neurodegeneration after treatment with the endotoxin. These findings suggest that PAs play a role in maintaining the neuronal integrity and cerebral homeostasis during immune insults (Soulet and Rivest, 2003). Although both TLR2 and TLR4 are implicated in the innate immune response of the intestinal epithelial cells to bacterial pathogens, only an increase in TLR2 transcripts with concomitant increase in epithelial barrier function was observed upon the ectopic expression of ODC and this increase was greatly reduced by DMFO treatment, an ODC inhibitor (Chen et al., 2007). Significant changes in the levels of TRL4 transcripts were not obtained by either decreasing or increasing the function of ODC suggesting that PAs specifically activate TLR2 expression that regulates the epithelial barrier function (Chen et al., 2007). Collectively, these results provide support to the hypothesis that PAs play a role in LPS-induced innate immunity, although more research is needed to establish the role(s) for various PAs present in the cellular milieu. The generation of ROS has been implicated in the activation of innate immunity of gastric epithelial cells and macrophages to counteract infection by Helicobacter pylori since H2O2 produced during the back conversion of Spd and Spm enhanced protection to gastric epithelial cells and macrophages against H. pylori (Gobert and Wilson, 2017). However, the ROS-mediated oxidative DNA damage and resulting mutations might also play a role in colonizing H. pylori to gastric niche (Gobert and Wilson, 2017). T-cells in aged mice exhibit decreased autophagy in the lysosomal degradation pathway leading to their decay. However, an antibacterial molecule from the hemocytes of the spider Acanthoscurria gomesiana has been reported to enhance the innate immune response in splenocytes by inducing IFN-γ and NO synthesis (Mafra et al., 2012).
Cognitive Impairments
PA effects on cognitive memory, including quinolinic acid (QA)-induced, lipopolysaccharide (LPS)-induced, amyloid peptide (A)-induced, and brain trauma-induced cognitive deficit in animal model systems have been reviewed recently (Guerra and Rubin, 2016). These studies have begun to provide further evidence that endogenous or dietary PAs can have either beneficial or deleterious effects on cognitive diseases depending upon physiological or pathological impairment of memory (Frühauf et al., 2015). Therefore, it appears that diets rich in PAs should have beneficial effects on “early” inflammation and memory impairment but may be harmful once the pathological conditions have set in.
Quinolinic Acid (QA)-Induced Memory Impairment
Huntington's disease (HD), a fatal genetic disorder, is associated with progressive breakdown of nerve cells in the brain causing memory deficits. Intrastriatal injection with quinolinic acid (QA) has provided animal model for this disease because it duplicates many histopathological and neurochemical symptoms and neurofunction loss of HD (DiFiglia, 1990). Intrastriatal injection with a 10 nmol Spm dose impaired object recognition after training in rodents, whereas a low dose of Spm (0.1 nmol) reverted the deleterious effect of a QA injection, attenuating QA-induced astrogliosis (Velloso et al., 2009). These results were interpreted to indicate that low Spm dose increases NMDAr activity in the striatum but an overdose decreases them (Velloso et al., 2009). Brain trauma-induced cognitive deficit increase in newly born astrocytes was reversed by feeding DFMO in the drinking water (Rosi et al., 2012). Interestingly, astrogliosis decreased during the reversal of QA-associated memory impairment (Velloso et al., 2009).
Lipopolysaccharide-induced neuroinflammation associated memory impairment LPS's are cell-wall components of gram-negative bacteria and induce neuroinflammation in the cerebral cortex and hippocampus. LPS-induced neuroinflammation that results in memory impairment is associated with spatial memory, contextual fear conditioning, and avoidance learning in rats and mice (Shaw et al., 2001; Lee et al., 2012). This type of neuroinflammation was found to be associated with increases in cytokines such as IFN-γ, IL-6, IL1-β, and TNFα levels. The intraperitoneally injected Spd (0.3 mg/kg) reversed the LPS-induced inflammation and memory impairment but not the LPS-induced increase in cytokines (Frühauf et al., 2015). Once again, involvement of Spd regulation of NMDAr activation was the reason given for these observed results (Li and Tsien, 2009). Glutamate and glycine/D-serine-mediated activation of NMDAr increases the flow of the positively charged ions through the cell membrane and, in the process, affects memory (Furukawa et al., 2005). However, the expression of these receptors is sensitive to the neuro-inflammatory challenges, affecting long-term NMDA-dependent potentiation in the hippocampus in the LPS-treated tissue (Min et al., 2009). Put, Spd, and Spm bind to the lower lobe of the N-terminal domain of GluN1 and GluN2B dimer interface, and allosterically activate NMDAr and impairing memory (Mony et al., 2011). Tenprodil, an inhibitor of the NMDAr, eliminates the protective effect of Spm, supporting this hypothesis. Collectively, these results, provide further evidence that NMDAr mediate the LPS-induced cognitive impairment (Frühauf et al., 2015).
Alzheimer and Parkinson Diseases
Alzheimer (AD) and Parkinson (PD) diseases are generally associated with aging and cause progressive decline of cognitive function. These severe neurological impairments are associated with accumulation of phosphorylated tau protein that forms neurofibrillary tangles and neurotoxic amyloid beta-peptide (Aβ), which is responsible for the formation of the senile plaques (Roberson and Mucke, 2006). The AD patients exhibit increase in the activity of ODC and PA levels in the brain, which had been implicated in the PAs role in both cognitive deficit and synaptic loss (Morrison et al., 1998; Inoue et al., 2013). Exposure of neuronal cell cultures to Aβ increased PA levels, NMDAr activation, and synaptic loss (Yatin et al., 2001). The intracerebral injection of Aβ induced cognitive impairment in experimental animals, which was reversed by NMDA antagonists, suggested a role in memory loss (Klyubin et al., 2011). Also, blocking the PA binding site in NDMAr either by arcaine or inhibiting PA synthesis by DFMO reversed the Aβ25-35-induced memory impairment in mice (Gross et al., 2013). Collectively, these studies provide evidence that PAs are deleterious to Aβ-accumulation and higher PA levels under these conditions cause cognitive decline (Inoue et al., 2013), a stipulation opposite to the effects of high PAs in improving learning and memory in naive animals. Parkinson's disease reduces expression of SAT1, a catabolic PA enzyme, and consequently higher PAs levels increased in patients. This increase in PAs levels has been implicated in cognitive reduction in Parkinson patients via NMDAr pathway (Lewandowski et al., 2010). Higher PA levels in Parkinson patients are also associated with the aggregation of α-synuclein, but its role in Parkinson's disease is not yet known.
Conclusions
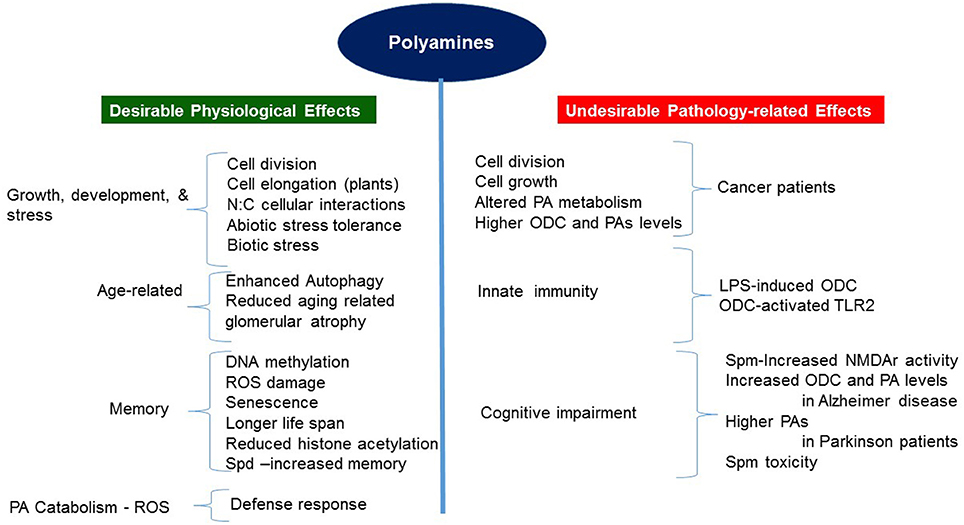
PAs play essential roles in a wide range of biochemical and physiological processes during growth and development of both mammals and plants, as also in their cellular responses to biotic and abiotic stresses (summarized in Figure 2). Our current understanding of the roles PAs play was intensified and emerged through genetic evidence obtained through the characterization of mutations in the PA biosynthetic pathway and analyzing molecular, biochemical and physiological changes in specific transgenic organisms altered in the levels of a particular PA or PAs (Mehta et al., 2002; Mattoo et al., 2006, 2010; Sriva et al., 2007; Sobolev et al., 2014; Kusano and Suzuki, 2015; Fatima et al., 2016; Goyal et al., 2016; Pegg, 2016). It has become apparent that PA function is dependent upon the cellular level of each PA, likely differentiable and specific to each diamine, triamine and tetramine (Put, Spd, and Spm, respectively) (Handa and Mattoo, 2010; Mattoo et al., 2010), and in many instances being temperature-dependent (Mattoo, 2014; Liu et al., 2015; Goyal et al., 2016). Further understanding of how living cells achieve PA homeostasis and which master regulators regulate their biosynthesis, interconversion, catabolism and conjugation to bring about desirable biologically active cellular concentrations to impact specific biological processes in normal and stressful environment(s) is necessary to fully appreciate their functions in different biological systems. In plants, for the most part, PAs seem to play positive roles in normal cellular functions, for instance, in increasing longevity, increasing pro-health carotenoids such as lycopene, recalling physiological memory, enhancing carbon and nitrogen resource allocation/signaling, as well as in plant development and responses to extreme environment.
Thus far, research has established that PAs generally boost physiological processes to reduce aging, stress-induced responses and loss of memory, but detrimentally influence pathology-related conditions such as cancer, Alzheimer and Parkinson diseases (Pegg, 2016). Genetic evidence for the positive PA roles in mammals is associated with a number of growth and developmental processes, including the behavioral aspects, and emerged through studies that characterized mice and human mutations with defective production of Spm (Lyon et al., 1986; Cason et al., 2003; Albert et al., 2013). Mechanistically, some functions of PAs are conserved, providing parallels between plants and humans. One such conserved parallel is the connection between PAs and the signaling TOR function in plants and humans (Ren et al., 2012; Zabala-Letona et al., 2017).
A beneficial role of dietary Spd in enhancing life span of various organisms and human cell lines tightens the connection between “wellness diets” and human health (Eisenberg et al., 2009). Emerging understanding of PA action should open up new vistas not only for human health but also the plant life. Interestingly, oral Spd-supplemented diet given to mice extended their life span and exerted cardioprotective effects, including reduced cardiac hypertrophy and preserving diastolic function in old mice (Eisenberg et al., 2016). A research survey based on dietary intake of Spd levels suggested a correlation of Spd levels with reduced blood pressure and a lower incidence of cardiovascular disease (Eisenberg et al., 2016). These positive features of higher PA Spd in enhancing quality of life both in animals/humans and plants alike augment well for the future development of PA-based therapies.
Author Contributions
AH: outlined the review; AH, TF, and AM: each wrote sections; AM: finalized the review.
Funding
AH's research is supported by USDA/NIFA Hatch IND011872. AM is supported by USDA/ARS intramural research project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agostinelli, E., Belli, F., Molinari, A., Condello, M., Palmigiani, P., Vedova, L. D., et al. (2006). Toxicity of enzymatic oxidation products of spermine to human melanoma cells (M14): sensitization by heat and MDL 72527. Biochim. Biophys. Acta 1763, 1040–1050. doi: 10.1016/j.bbamcr.2006.07.014
Ahou, A., Martignago, D., Alabdallah, O., Tavazza, R., Stano, P., Macone, A., et al. (2014). A plant spermine oxidase/dehydrogenase regulated by the proteasome and polyamines. J. Exp. Bot. 65, 1585–1603. doi: 10.1093/jxb/eru016
Aitken, A. (1996). 14-3-3 and its possible role in co-ordinating multiple signaling pathways. Trends Cell Biol. 6, 341–347. doi: 10.1016/0962-8924(96)10029-5
Albert, J., Schwartz, C., Boerkoel, C., and Stevenson, R. (2013). “Snyder-robinson syndrome,” in Gene Review (Seattle, WA: University of Washington), 1993–2018.
Alcazar, R., Altabella, T., Marco, F., Bortolotti, C., Reymond, M., Koncz, C., et al. (2010b). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249. doi: 10.1007/s00425-010-1130-0
Alcázar, R., Marco, F., Cuevas, J. C., Patron, M., Ferrando, A., Carrasco, P., et al. (2006). Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 28, 1867–1876. doi: 10.1007/s10529-006-9179-3
Alcazar, R., Planas, J., Saxena, T., Zarza, X., Bortolotti, C., Cuevas, J., et al. (2010a). Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants overexpressing the homologous arginine decarboxylase 2 gene. Plant Physiol. Biochem. 48, 547–552. doi: 10.1016/j.plaphy.2010.02.002
Ali, M. A., Poortvliet, E., Strömberg, R., and Yngve, A. (2011). Polyamines in foods: development of a food database. Food Nutr. Res. 55:5572. doi: 10.3402/fnr.v55i0.5572
Anderson, D. J., Crossland, J., and Shaw, G. G. (1975). The actions of spermidine and spermine on the central nervous system. Neuropharmacology 14, 571–577. doi: 10.1016/0028-3908(75)90123-9
Andersson, G., and Heby, O. (1972). Polyamine and nucleic acid concentrations in Ehrlich ascites carcinoma cells and liver of tumor-bearing mice at various stages of tumor growth. J. Natl Cancer Inst. 48, 165–172.
Angelini, R., Tisi, A., Rea, G., Chen, M. M., Botta, M., Federico, R., et al. (2008). Involvement of polyamine oxidase in wound healing. Plant Physiol. 146, 162–177. doi: 10.1104/pp.107.108902
Anwar, R., Mattoo, A. K., and Handa, A. K. (2015). “Polyamine interactions with plant hormones: crosstalk at several levels,” in Polyamines A: Universal Molecular Nexus for Growth, Survival and Specialized Metabolism eds T. Kusano, H. Suzuki (New York, NY: Springer), 267–302
Artignan, X., Miglianico, L., Bligny, D., Abraham, C., Moulinoux, J. P., and Cipolla, B. (2012). Combination of a polyamine-free oral nutritional supplement (ONS) with docetaxel in castrate-resistant prostate cancer (CRPC) patients (pts): A phase II trial.). J. Clin. Oncol. 30, 67–67. doi: 10.1200/jco.2012.30.5_suppl.67
Bacchi, C. J., and Yarlett, N. (2002). Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2, 553–563. doi: 10.2174/1389557023405549
Bachrach, U., and Wang, Y. C. (2002). Cancer therapy and prevention by green tea: role of ornithine decarboxylase. Amino Acids 22, 1–13. doi: 10.1007/s726-002-8197-9
Bardócz, S., Grant, G., Brown, D. S., Ralph, A., and Pusztai, A. (1993). Polyamines in food – implications for growth and health. J. Nutr. Biochem. 4, 66–71. doi: 10.1016/0955-2863(93)90001-D
Baronas, V. A., and Kurata, H. T. (2014). Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 5:325. doi: 10.3389/fphys.2014.00325
Bauer, G. A., Bazzaz, F. A., Minocha, R., Long, S., Magill, A., Aber, J., et al. (2004). Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in NE United States. Forest Ecol. Manage. 196, 173–186. doi: 10.1016/j.foreco.2004.03.032
Bitrián, M., Zarza, X., Altabella, T., Tiburcio, A. F., and Alcázar, R. (2012). Polyamines under abiotic stress: metabolic crossroads and hormonal crosstalks in plants. Metabolites 2, 516–528. doi: 10.3390/metabo2030516
Bonini, J. S., Da Silva, W. C., Da Silveira, C. K., Kohler, C. A., Izquierdo, I., and Cammarota, M. (2011). Histamine facilitates consolidation of fear extinction. Int. J. Neuropsychopharmacol. 14, 1209–1217. doi: 10.1017/S1461145710001501
Bowie, D., and Mayer, M. L. (1995). Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 doi: 10.1016/0896-6273(95)90049-7
Bridges, D., and Moorhead, G. B. G. (2004). 14-3-3 proteins: a number of functions for a numbered protein. Sci. STKE 296:re10. doi: 10.1126/stke.2422004re10
Brubaker, S. W., Bonham, K. S., Zanoni, I., and Kagan, J. C. (2015). Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290. doi: 10.1146/annurev-immunol-032414-112240
Cai, G., Sobieszczuk-Nowicka, I. S., Aloisi, I., Fattorini, L., Serafini-Fracassini, D., and Del Duca, S. (2015). Polyamines are common players in different facets of plant programmed cell death. Amino Acids 47, 27–44. doi: 10.1007/s00726-014-1865-1
Camera, K., Mello, C. F., Ceretta, A. P., and Rubin, M. A. (2007). Systemic administration of polyaminergic agents modulate fear conditioning in rats. Psychopharmacology (Berl). 192, 457–464. doi: 10.1007/s00213-007-0734-y
Capell, T., Bassie, L., and Christou, P. (2004). Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. U.S.A. 101, 9909–9914. doi: 10.1073/pnas.0306974101
Caraglia, M., Park, M. H., Wolff, E. C., Marra, M., and Abbruzzese, A. (2013). eIF5A isoforms and cancer: two brothers for two functions? Amino Acids 44, 103–109. doi: 10.1007/s00726-011-1182-x
Casero Jr, R. A., and Marton, L. J. (2007). Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Disc. 6, 373–390. doi: 10.1038/nrd2243
Cason, A. L., Ikeguchi, Y., Skinner, C., Wood, T. C., Holden, K. R., Lubs, H. A., et al. (2003). X-Linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur. J. Hum. Genet. 11, 937–944. doi: 10.1038/sj.ejhg.5201072
Cassol, T., and Mattoo, A. K. (2003). “Do polyamines and ethylene interact to regulate plant growth, development and senescence?,” in Molecular Insight in Plant Biology, eds P. Nath, A. K. Mattoo, S. A. Ranade, and J. H. Weil (Enfield, NH: Science Publishers Inc.), 121–132.
Chamaillard, L., Catros-Quemener, V., Delcros, J. G., Bansard, J. Y., Havouis, R., Desury, D., et al. (1997). Polyamine deprivation prevents the development of tumour-induced immunesuppression. Br. J. Cancer 76, 365–370. doi: 10.1038/bjc.1997.391
Chen, J., Rao, J. N., Zou, T., Liu, L., Marasa, B. S., Xiao, L., et al. (2007). Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 293, 568–576. doi: 10.1152/ajpgi.00201.2007
Cipolla, B. G., Havouis, R., and Moulinoux, J. P. (2007). Polyamine contents in current foods: a basis for polyamine reduced diet and a study of its longterm observance and tolerance in prostate carcinoma patients. Amino Acids 33, 203–212. doi: 10.1007/s00726-007-0524-1
Cirilo, M. P. G., Coelho, A. F. S., Araujo, C. M., Goncalves, F. R. B., Nogueira, F. D., and Gloria, M. B. A. (2003). Profile and levels of bioactive amines in green and roasted coffee. Food Chem. 82, 397–402. doi: 10.1016/S0308-8146(02)00560-5
Coleman, C. S., Hu, G., and Pegg, A. E. (2004). Putrescine biosynthesis in mammalian tissues. Biochem. J. 379, 849–855. doi: 10.1042/bj20040035
Cona, A., Cenci, F., Cervelli, M., Federico, R., Mariottini, P., Moreno, S., et al. (2003). Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol. 131, 803–813. doi: 10.1104/pp.011379
Cona, A., Rea, G., Angelini, R., Federico, R., and Tavladoraki, P. (2006). Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11, 80–88. doi: 10.1016/j.tplants.2005.12.009
Conway, E. L. (1998). Brain lesions and delayed water maze learning deficits after intracerebroventricular spermine. Brain Res. 800, 10–20 doi: 10.1016/S0006-8993(98)00487-9
Da Rosa, M. M., Mello, C. F., Camera, K., Ceretta, A. P., Ribeiro, D. A., Signor, C., et al. (2012). Opioid mechanisms are involved in the disruption of arcaine-induced amnesia by context pre-exposure. Neurobiol. Learn. Mem. 97, 294–300. doi: 10.1016/j.nlm.2012.02.002
Deretic, V., Saitoh, T., and Akira, S. (2013). Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13, 722–737. doi: 10.1038/nri3532
Dicáková, Z., Paulsen, P., Bystrický, P., Soko, J., and Laczkóová, S. (2003). “Determination of biogenic amines and free amino acids in game meat during storage,” in Hygiena Alimentorum, Vol. XXIV ed. P. Bystrický, J. Nagy, and D. Máté (Košice: University of Veterinary Medicine), 97–99.
DiFiglia, M. (1990). Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci. 13, 286–289. doi: 10.1016/0166-2236(90)90111-M
Edwards, R. A., Dainty, R. H., and Hibbard, C. M. (1983). The relationship of bacterial numbers and types to diamine concentration in fresh and aerobically stored beef, pork and lamb. J. Food Technol. 18, 777–788. doi: 10.1111/j.1365-2621.1983.tb00316.x
Eisenberg, T., Abdellatif, M., Schroeder, S., Primessnig, U., Stekovic, S., Pendl, T., et al. (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 12, 1428–1438. doi: 10.1038/nm.4222
Eisenberg, T., Knauer, H., Schauer, A., Buttner, S., Ruckenstuhl, C., Carmona-Gutierrez, D., et al. (2009). Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314. doi: 10.1038/ncb1975
Eliassen, K. A., Reistad, R., Risoen, U., and Ronning, H. F. (2002). Dietary polyamines. Food Chem. 78, 273–280. doi: 10.1016/S0308-8146(01)00405-8
Farriol, M., Venereo, Y., Orta, X., Company, C., Gomez, P., Delgado, G., et al. (2004). Ingestion of antioxidants and polyamines in patients with severe burns. Nutr. Hosp. 19, 300–304.
Fatima, T., Sobolev, A. P., Teasdale, J. R., Kramer, M., Bunce, J., Handa, A. K., et al. (2016). Fruit metabolite networks in engineered and non-engineered tomato genotypes reveal fluidity in a hormone and agroecosystem specific manner. Metabolomics 12:103. doi: 10.1007/s11306-016-1037-2
Fincato, P., Moschou, P. N., Spedaletti, V., Tavazza, R., Angelini, R., Federico, R., et al. (2011). Functional diversity inside the Arabidopsis polyamine oxidase gene family. J. Exp. Bot. 62, 1155–1168. doi: 10.1093/jxb/erq341
Foyer, C. H., and Noctor, G. (2002). Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism. Boston, MA: Kluwer Academic Publlishers.
Frühauf, P. K., Ineu, R. P., Tomazi, L., Duarte, T., Mello, C., and Rubin, M. A. (2015). Spermine reverses lipopolysaccharide-induced memory deficit in mice. J. Neuroinflamm. 12, 3. doi: 10.1186/s12974-014-0220-5
Furukawa, H., Singh, S. K., Mancusso, R., and Gouaux, E. (2005). Subunit arrangement and function in NMDA receptors. Nature 438, 185–192. doi: 10.1038/nature04089
Garufi, A., Visconti, S., Camoni, L., and Aducci, P. (2007). Polyamines as physiological regulators of 14-3-3 interaction with the plant plasma membrane H+- ATPase. Plant Cell Physiol. 48, 434–440. doi: 10.1093/pcp/pcm010
Ghuge, S. A., Carucci, A., Rodrigues Pousada, R. A., Tisi, A., Franchi, S., et al. (2015). The apoplastic copper AMINE OXIDASE1 mediates jasmonic acid-induced protoxylem differentiation in Arabidopsis roots. Plant Physiol. 168, 690–707. doi: 10.1104/pp.15.00121
Giardiello, F. M., Hamilton, S. R., Hylind, L. M., Yang, V. W., Tamez, P., Casero, R. A. Jr., et al. (1997). Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 57, 199–120.
Gilmour, S. K. (2007). Polyamines and nonmelanoma skin cancer. Toxicol Appl. Pharmacol. 224, 249–256. doi: 10.1016/j.taap.2006.11.023
Gobert, A. P., and Wilson, K. T. (2017). Polyamine- and NADPH-dependent generation of ROS during Helicobacter pylori infection: a blessing in disguise. Free Radic. Biol. Med. 105, 16–27. doi: 10.1016/j.freeradbiomed.2016.09.024
Gomes, G. M., Mello, C. F., da Rosa, M. M., Bochi, G. V., Ferreira, J., Barron, S., et al. (2010). Polyaminergic agents modulate contextual fear extinction in rats. Neurobiol. Learn. Mem. 93, 589–595. doi: 10.1016/j.nlm.2010.02.007
Goyal, R. K., Fatima, T., Topuz, M., Bernadec, A., Sicher, R., Handa, A. K., et al. (2016). Pathogenesis-related protein 1b1 (PR1b1) is a major tomato fruit protein responsive to chilling temperature and upregulated in high polyamine transgenic genotypes. Front. Plant Sci. 7:901. doi: 10.3389/fpls.2016.00901
Grimes, H. D., Slocum, R. D., and Boss, W. F. (1986). α-Difluoromethylarginine treatment inhibits protoplast fusion in fusogenic wild-carrot protoplasts. Biochim. Biophys. Acta 886, 130–134. doi: 10.1016/0167-4889(86)90218-1
Gross, J. A., Fiori, L. M., Labonte, B., Lopez, J. P., and Turecki, G. (2013). Effects of promotermethylation on increased expression of polyamine biosynthetic genes in suicide. J. Psychiatr. Res. 47 513–519. doi: 10.1016/j.jpsychires.2012.11.016
Guerra, G. P., and Rubin, M. A. (2016). Mello Modulation CF of learning and memory by natural polyamines. Pharmacol. Res. 112, 99–118. doi: 10.1016/j.phrs.2016.03.023
Gupta, K., Sengupta, A., Chakraborty, M., and Gupta, B. (2016). Hydrogen Peroxide and Polyamines act as double edged swords in plant abiotic stress responses. Front. Plant Sci. 7:1343. doi: 10.3389/fpls.2016.01343
Gupta, V. K., Scheunemann, L., Eisenberg, T., Mertel, S., Bhukel, A., Koemans, T. S., et al. (2013). Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 16, 1453–1460. doi: 10.1038/nn.3512
Hamana, K., Niitsu, M., and Samejima, K. (1998). Unusual polyamines in aquatic plants: the occurrence of homospermidine, norspermidine, thermospermine, norspermine, aminopropylhomospermidine, bis(aminopropyl)ethanediamine, and methylspermidine. Can. J. Bot. 76, 130–133. doi: 10.1139/b97-175
Handa, A. K., and Mattoo, A. K. (2010). Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Biochem. 48, 540–546. doi: 10.1016/j.plaphy.2010.02.009
He, L., Ban, Y., Inoue, H., Matsuda, N., Liu, J., and Moriguchi, T. (2008). Enhancement of spermidine content and antioxidant capacity in transgenic pear shoots over expressing apples spermidine synthase in response to salinity and hyperosmosis. Phytochemistry 69, 2133–2141. doi: 10.1016/j.phytochem.2008.05.015
Hernandez-Jover, T., Izquierdo-Pulido, M., Veciana-Nogues, M. T., Marine-Font, A., and Vidal-Carou, M. C. (1997). Biogenic amine and polyamine contents in meat and meat products. J. Agric. Food Chem. 45, 2098–2102. doi: 10.1021/jf960790p
Hibino, H., Inanobe, A., Furutani, K., Murakami, S., Findlay, I., and Kurachi, Y. (2010). Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366. doi: 10.1152/physrev.00021.2009
Liu, J-H., Wang, W., Wu, H., Gong, X., and Moriguchi, T. (2015). Polyamines function in stress tolerance: from synthesis to regulation. Front. Plant Sci. 6:827. doi: 10.3389/fpls.2015.00827
Igarashi, K., and Kashiwagi, K. (2010). Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiol. Biochem. 48, 506–512. doi: 10.1016/j.plaphy.2010.01.017
Inoue, K., Tsutsui, H., Akatsu, H., Hashizume, Y., Matsukawa, N., Yamamoto, T., et al. (2013). Metabolic profiling of Alzheimer's disease brains. Sci. Rep. 3:2364. doi: 10.1038/srep02364
Janne, J., Alhonen, L., Pietila, M., and Keinanen, T. A. (2004). Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. 271, 877–894. doi: 10.1111/j.1432-1033.2004.04009.x
Jell, J., Merali, S., Hensen, M. L., Mazurchuk, R., Spernyak, J. A., Diegelman, P., et al. (2007). Genetically altered expression of spermidine/spermine N1-acetyl-transferase affects fat metabolism in mice via acetyl-CoA. J. Biol. Chem. 282, 8404–8413. doi: 10.1074/jbc.M610265200
Kalač, P. (2014). Health effects and occurrence of dietary polyamines: a review for the period 2005–mid 2013. Food Chem. 161, 27–39. doi: 10.1016/j.foodchem.2014.03.102
Kalac, P., and Krausova, P. (2005). A review of dietary polyamines: formation, implications for growth and health and occurrence in foods. Food Chem. 90, 219–230. doi: 10.1016/j.foodchem.2004.03.044
Kasinathan, V., and Wingler, A. (2004). Effect of reduced arginine decarboxylate activity on salt tolerance and on polyamine formation during salt stress in Arabidopsis thaliana. Plant Physiol. 121, 101–107. doi: 10.1111/j.0031-9317.2004.00309.x
Kasukabe, Y., He, L., Nada, K., Misawa, S., Ihara, I., and Tachibana, S. (2004). Over expression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 45, 712–722. doi: 10.1093/pcp/pch083
Kasukabe, Y., He, L., Watakabe, Y., Otani, M., Shimada, T., and Tachibana, S. (2006). Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol. 23, 75–83. doi: 10.5511/plantbiotechnology.23.75
Kaur-Sawhney, R., Tiburcio, A. F., Altabella, T., and Galston, A. W. (2003). Polyamines in plants: an overview. J. Cell. Mol. Biol. 2, 1–12. doi: 10.4236/ajps.2013.45A013
Kim, D. W., Watanabe, K., Murayama, C., Izawa, S., Niitsu, M., Michael, A. J., et al. (2014). Polyamine oxidase 5 regulates Arabidopsis growth through thermospermine oxidase activity. Plant Physiol. 165, 1575–1590. doi: 10.1104/pp.114.242610
Kim, J. H., Ahn, H. J., Kim, D. H., Jo, C., Yook, H. S., Park, H.-J., et al. (2003). Irradiation effects on biogenic amines in Korean fermented soybean paste during fermentation. J. Food Sci. 68, 80–84. doi: 10.1111/j.1365-2621.2003.tb14118.x
Kishi, A., Ohno, M., and Watanabe, S. (1998a). Concurrent activation of hippocampal glycine and polyamine sites of the N-methyl-d-aspartate receptor synergistically reverses working memory deficits in rats. Neurosci. Lett. 257, 131–134 doi: 10.1016/S0304-3940(98)00824-6
Kishi, A., Ohno, M., and Watanabe, S. (1998b). Spermidine, a polyamine site agonist, attenuates working memory deficits caused by blockade of hippocampal muscarinic receptors and mGluRs in rats. Brain Res. 793, 311–314. doi: 10.1016/S0006-8993(98)00179-6
Klyubin, I., Wang, Q., Minard, N. R., Irving, E. A., Upton, J., Hofmeister, J., et al. (2011). Protection against Abeta-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol. Aging 32, 614–623. doi: 10.1016/j.neurobiolaging.2009.04.005
Kozova, M., Kalac, P., and Pelikanova, T. (2009a). Contents of biologically active polyamines in chicken meat, liver, heart and skin after slaughter and their changes during meat storage and cooking. Food Chem. 116, 419–425. doi: 10.1016/j.foodchem.2009.02.057
Kozova, M., Kalac, P., and Pelikanova, T. (2009b). Changes in the content of biologically active polyamines during beef loin storage and cooking. Meat Sci. 81, 607–611. doi: 10.1016/j.meatsci.2008.10.018
Kumar, A., Taylor, M. A., Arif, S. A. M., and Davies, H. V. (1996). Potato plants expressing antisense and sense S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. Plant J. 9, 147–158. doi: 10.1046/j.1365-313X.1996.09020147.x
Kusano, T., Berberich, T., Tateda, C., and Takahashi, Y. (2008). Polyamines: essential factors for growth and survival. Planta 228, 367–381. doi: 10.1007/s00425-008-0772-7
Kusano, T., and Suzuki, H. (2015). Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism. New York, NY: Springer.
Kusano, T., Yamaguchi, K., Berberich, T., and Takahashi, Y. (2007). Advances in polyamine research. Curr. Top. Plant Res. 120, 345–350. doi: 10.1007/s10265-007-0074-3
Lagishetty, C. V., and Naik, S. R. (2008). Polyamines: potential anti-inflammatory agents and their possible mechanism of action. Ind. J. Pharmacol. 40, 121–125. doi: 10.4103/0253-7613.42305
Lamkanfi, M., and Dixit, V. M. (2014). Mechanisms and functions of inflammasomes. Cell 157, 1013–1022. doi: 10.1016/j.cell.2014.04.007
Lavizzari, T., Teresa Veciana-Nogues, M., Bover-Cid, S., Marine-Font, A., and Carmen Vidal-Carou, M. (2006). Improved method for the determination of biogenic amines and polyamines in vegetable products by ion-pair high-performance liquid chromatography. J Chromatogr. A. 1129, 67–72. doi: 10.1016/j.chroma.2006.06.090
Lee, J., Michael, A. J., Martynowski, D., Goldsmith, E. J., and Phillips, M. A. (2007). Phylogenetic diversity and the structural basis of substrate specificity in the beta/alpha-barrel fold basic amino acid decarboxylases. J. Biol. Chem. 282, 27115–27125. doi: 10.1074/jbc.M704066200
Lee, J., Sperandio, V., Frantz, D. E., Longgood, J., Camilli, A., Phillips, M. A., et al. (2009). An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae. J. Biol Chem. 284, 9899–9907. doi: 10.1074/jbc.M900110200
Lee, Y. J., Choi, D. Y., Choi, I. S., Kim, K. H., Kim, Y. H., Kim, H. M., et al. (2012). Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J. Neuroinflammat. 9:35. doi: 10.1186/1742-2094-9-35
Lewandowski, N. M., Ju, S., Verbitsky, M., Ross, B., Geddie, M. L., Rockenstein, E., et al. (2010). Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 107, 16970–16975. doi: 10.1073/pnas.1011751107
Li, F., and Tsien, J. Z. (2009). Memory and the NMDA receptors. N. Engl. J. Med. 361, 302–303. doi: 10.1056/NEJMcibr0902052
Lorenz, B., Francis, F., Gempel, K., Böddrich, A., Josten, M., Schmahl, W., et al. (1998). Spermine deficiency in Gy mice caused by deletion of the spermine synthase gene. Hum. Mol. Genet. 7, 541–547. doi: 10.1093/hmg/7.3.541
Lyon, M. F., Scriver, C. R., Baker, L. R., Tenenhouse, H. S., Kronick, J., and Mandla, S. (1986). The Gy mutation: another cause of X-linked hypophosphatemia in mouse. Proc. Natl. Acad. Sci. U.S.A. 83, 4899–4903 doi: 10.1073/pnas.83.13.4899
Mafra, D. G., da Silva, P. I. Jr., Galhardo, C. S., Nassar, R., Daffre, S., Sato, M. N., et al. (2012). The spider acylpolyamine Mygalin is a potent modulator of innate immune responses. Cell Immunol. 275, 5–11. doi: 10.1016/j.cellimm.2012.04.003
Majumdar, R., Shao, L., Minocha, R., Long, S., and Minocha, S. C. (2013). Ornithine: the overlooked molecule in the regulation of polyamine metabolism. Plant Cell Physiol. 54, 990–1004. doi: 10.1093/pcp/pct053
Mandal, A., Mandal, S., and Park, M. H. (2014). Genome-wide analyses and functional classification of proline repeat-rich proteins: potential role of eIF5A in eukaryotic evolution. PLoS ONE. 9:e111800. doi: 10.1371/journal.pone.0111800
Manni, A., Grove, R., Kunselman, S., and Demers, L. (1995). Involvement of the polyamine pathway in breast cancer progression. Cancer Lett. 92, 49–57. doi: 10.1016/0304-3835(95)03763-M
Marina, M., Sirera, F. V., Rambla, J. L., Gonzalez, M. E., Blázquez, M. A., Carbonell, J., et al. (2013). Thermospermine catabolism increases Arabidopsis thaliana resistance to Pseudomonas viridiflava. J. Exp. Bot. 64, 1393–1402. doi: 10.1093/jxb/ert012
Martin-Tanguy, J. (2001). Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul. 34, 135–148. doi: 10.1023/A:1013343106574
Mathews, M. B., and Hershey, J. W. (2015). The translation factor eIF5A and human cancer. Biochim. Biophys. Acta 1849, 836–844. doi: 10.1016/j.bbagrm.2015.05.002
Mattoo, A. K. (2014). Translational research in agricultural biology—enhancing crop resistivity against environmental stress alongside nutritional quality. Front. Chem. 2:30. doi: 10.3389/fchem.2014.00030
Mattoo, A. K., and Handa, A. K. (2008). Higher polyamines restore and enhance metabolic memory in ripening fruit. Plant Sci. 174, 386–393. doi: 10.1016/j.plantsci.2008.01.011
Mattoo, A. K., Minocha, S. C., Minocha, R., and Handa, A. K. (2010). Polyamines and cellular metabolism in plants: transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids. 38, 405–413. doi: 10.1007/s00726-009-0399-4
Mattoo, A. K., Sobolev, A. P., Neelam, A., Goyal, R. K., Handa, A. K., and Segre, A. L. (2006). NMR spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions. Plant Physiol. 142, 1759–1770. doi: 10.1104/pp.106.084400
Mattoo, A. K., Upadhyay, R. K., and Rudrabhatla, S. (2015). “Abiotic stress in crops: candidate genes, osmolytes, polyamines, and biotechnological intervention,” in Elucidation of Abiotic Stress Signaling in Plants, ed G. K. Pandey (New York, NY: Springer Science+Business Media), 415–437.
Medzhitov, R. (2001). Toll like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145. doi: 10.1038/35100529
Medzhitov, R. (2009). Approaching the asymptote: 20 years later. Immunity 30, 766–775. doi: 10.1016/j.immuni.2009.06.004
Mehta, R. A. T., Cassol, N., Li, N., Ali Handa, A. K., and Mattoo, A. K. (2002). Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality and vine life. Nat. Biotech. 20, 613–618. doi: 10.1038/nbt0602-613
Meyer, R. A. Jr., Henley, C. M., Meyer, M. H., Morgan, P. L., McDonald, A. G., Mills, C., et al. (1998). Partial deletion of both the spermine synthase gene and the Pex gene in the x-linked hypophosphatemic, Gyro (Gy) mouse. Genomics 48, 289–295 doi: 10.1006/geno.1997.5169
Min, S. S., Quan, H. Y., Ma, J., Han, J. S., Jeon, B. H., and Seol, G. H. (2009). Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal CA1 area. Neurosci. Lett. 456, 20–24. doi: 10.1016/j.neulet.2009.03.079
Minocha, R., Majumdar, R., and Minocha, S. C. (2014). Polyamines and abiotic stress in plants: a complex relationship. Front. Plant Sci. 5:175. doi: 10.3389/fpls.2014.00175
Minois, N. (2014). Molecular basis of the anti-aging effects of spermidine and other natural polyamines - a mini-review. Gerontology 60, 319–326. doi: 10.1159/000356748
Minois, N., Carmona-Gutierrez, D., and Madeo, F. (2011). Polyamines in aging and disease. Aging 3, 716–732. doi: 10.18632/aging.100361
Mo, H. J., Sun, Y. X., Zhu, X. L., Wang, X. F., Zhang, Y., Yang, J., et al. (2016). Cotton S-adenosylmethionine decarboxylase-mediated spermine biosynthesis is required for salicylic acid- and leucine-correlated signaling in the defense response to Verticillium dahliae. Planta 243, 1023–1039. doi: 10.1007/s00425-015-2463-5
Mo, H., Wang, X., Zhang, Y., Zhang, G., Zhang, J., and Ma, Z. (2015). Cotton polyamine oxidase is required for spermine and camalexin signalling in the defence response to Verticillium dahliae. Plant J. 83, 962–975. doi: 10.1111/tpj.12941
Mony, L., Zhu, S., Carvalho, S., and Paoletti, P. (2011). Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J. 30, 3134–3146. doi: 10.1038/emboj.2011.203
Morris, R. G. M., Anderson, E., Lynch, G. S., and Baudry, M. (1986). Selective impairment of learning and blockade of long-term poten- tiation byanN-methyl-D-aspartate receptor antagonists, AP5. Nature 319, 774–776. doi: 10.1038/319774a0
Morrison, L. D., Cao, X. C., and Kish, S. J. (1998). Ornithine decarboxylase in human brain: influence of aging, regional distribution, and Alzheimer's disease. J. Neurochem. 71, 288–294. doi: 10.1046/j.1471-4159.1998.71010288.x
Morselli, E., Maiuri, M. C., Markaki, M., Megalou, E., Pasparaki, A., Palikaras, K., et al. (2010). Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Disease 1:10. doi: 10.1038/cddis.2009.8
Moschou, P. N., Paschalidis, K. A., Delis, I. D., Andriopoulou, A. H., Lagiotis, G. D., Yakoumakis, D. I., et al. (2008). Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell. 20, 1708–1724. doi: 10.1105/tpc.108.059733
Moschou, P. N., and Roubelakis-Angelakis, K. A. (2014). Polyamines and programmed cell death. J. Exp. Bot. 65, 1285–1296. doi: 10.1093/jxb/ert373
Moschou, P. N., Sarris, P. F., Skandalis, N., Andriopoulou, A. H., Paschalidis, K. A., Panopoulos, N. J., et al. (2009). Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiol. 149, 1970–1981. doi: 10.1104/pp.108.134932
Nambeesan, S., Abu-Qamar, S., Laluk, K., Mattoo, A. K., Mickelbart, M. V., Ferruzzi, M. G., et al. (2012). Polyamines attenuate ethylene-mediated defense responses to abrogate resistance to Botrytis cinerea in tomato. Plant Physiol. 158, 1034–1045. doi: 10.1104/pp.111.188698
Nambeesan, S., Datsenka, T., Ferruzzi, M. G., Malladi, A., Mattoo, A. K., and Handa, A. K. (2010). Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J. 63, 836–847. doi: 10.1111/j.1365-313X.2010.04286.x
Neelam, A., Cassol, T., Mehta, R. A., Abdul-Baki, A. A., Sobolev, A., Goyal, R. K., et al. (2008). A field-grown transgenic tomato line expressing higher levels of polyamines reveals legume cover crop mulch-specific perturbations in fruit phenotype at the levels of metabolite profiles, gene expression and agronomic characteristics. J. Exp. Bot. 59:2337–2346. doi: 10.1093/jxb/ern100
Nishibori, N., Fujihara, S., and Akatuki, T. (2007). Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 100, 491–497. doi: 10.1016/j.foodchem.2005.09.070
Nishimura, K., Lee, S. B., Park, J. H., and Park, M. H. (2012). Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids 42, 703–710. doi: 10.1007/s00726-011-0986-z
Nishimura, K., Shiina, R., Kashiwagi, K., and Igarashi, K. (2006). Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 139, 81–90. doi: 10.1093/jb/mvj003
Noh, E. W., and Minocha, S. C. (1994). Expression of a human S-adenosylmethionine decarboxylase cDNA in transgenic tobacco and its effects on polyamine biosynthesis. Transgenic Res. 3, 26–35. doi: 10.1007/BF01976024
Nowotarski, S. L., Woster, P. M., and Casero, R. A. Jr. (2013). Polyamines and cancer: implications for chemotherapy and chemoprevention. Expert Rev. Mol. Med. 15:e3. doi: 10.1017/erm.2013.3
Okamoto, A., Sugi, E., Koizumi, Y., Yanagida, F., and Udaka, S. (1997). Polyamine content of ordinary foodstuffs and various fermented foods. Biosci. Biotechnol. Biochem. 61, 1582–1584. doi: 10.1271/bbb.61.1582
Page, A. F., Cseke, L. J., Minocha, R., Turlapati, S. A., Podila, G. K., Ulanov, A., et al. (2016). Genetic manipulation of putrescine biosynthesis reprograms the cellular transcriptome and the metabolome. BMC Plant Biol. 16:113. doi: 10.1186/s12870-016-0796-2
Pällmann, N., Braig, M., Sievert, H., et al. (2015). Biological relevance and therapeutic potential of the hypusine modification system. J. Biol. Chem. 290, 18343–18360. doi: 10.1074/jbc.M115.664490
Pal, M., Szalai, G., and Janda, T. (2015). Speculation: polyamines are important in abiotic stress signaling. Plant Sci. 237, 16–23. doi: 10.1016/j.plantsci.2015.05.003
Palm, N. W., and Medzhitov, R. (2009). Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 227, 221–233. doi: 10.1111/j.1600-065X.2008.00731.x
Park, M. H. (2006). The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 139, 161–169. doi: 10.1093/jb/mvj034
Paschalidis, K. A., and Roubelakis-Angelakis, K. A. (2005). Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation. Plant Physiol. 138, 142–152. doi: 10.1104/pp.104.055483
Pathak, M. R., Teixeira da Silva, J. A., and Wani, S. H. (2014). Polyamines in response to abiotic stress tolerance through transgenic approaches. GM Crops Food 5, 87–96. doi: 10.4161/gmcr.28774
Pegg, A. E. (2016). Functions of polyamines in mammals. J. Biol. Chem. 291, 14904–14912. doi: 10.1074/jbc.R116.731661
Perez-Leal, O., and Merali, S. (2011). Regulation of polyamine metabolism by translational control. Amino acids. 42, 611–617. doi: 10.1007/s00726-011-1036-6
Pfeffer, L. M., Yang, C. H., Murti, A., McCormack, S. A., Viar, M. J., Ray, R. M., et al. (2001). Polyamine depletion induces rapid NF-kB activation in IEC-6 cells. J. Biol. Chem. 276, 45909–45913. doi: 10.1074/jbc.M108097200
Pignatti, C., Tantini, B., Stefanelli, C., and Flamigni, F. (2004). Signal transduction pathways linking polyamines to apoptosis. Amino Acids 27, 359–365. doi: 10.1007/s00726-004-0115-3
Pirnes-Karhu, S., Sironen, R., Alhonen, L., and Uimari, A. (2012). Lipopolysaccharide-induced anti-inflammatory acute phase response is enhanced in spermidine /spermine N1-acetyltransferase (SSAT) overexpressing mice. Amino Acids. 42, 473–484. doi: 10.1007/s00726-011-1026-8
Planas-Portell, J., Gallart, M., Tiburcio, A. F., and Altabella, T. (2013). Copper containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 13:109. doi: 10.1186/1471-2229-13-109
Prabhavathi, V. R., and Rajam, M. V. (2007). Polyamine accumulation in transgenic eggplant enhances tolerance to multiple abiotic stresses and fungal resistance. Plant Biotechnol. 24, 273–282. doi: 10.5511/plantbiotechnology.24.273
Proud, C. G. (2004). Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr. Top. Microbiol. Immunol. 279, 215–244. doi: 10.1007/978-3-642-18930-2_13
Provan, F., Aksland, L. M., Meyer, C., and Lillo, C. (2000). Deletion of the nitrate reductase N-terminal domain still allows binding of 14-3-3 proteins but affects their inhibitory properties. Plant Physiol. 123, 57–764. doi: 10.1104/pp.123.2.757
Pucciarelli, S., Moreschini, B., Micozzi, D., De Fronzo, G. S., Carpi, F. M., Polzonetti, V., et al. (2012). Spermidine and spermine are enriched in whole-blood of nona/centenarians. Rejuv. Res. 15, 590–595. doi: 10.1089/rej.2012.1349
Ransom, R. W., and Stec, N. L. (1988). Cooperative modulation of [3H]MK-801 binding to the N-methyl-d-aspartate receptor-ion channel complex by l-glutamate glycine, and polyamines. J. Neurochem. 51, 830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x
Ren, M., Venglat, P., Qiu, S., Feng, L. I., Cao, Y., Wang, E., et al. (2012). Target of rapamycin signaling regulates metabolism, growth, and life span in Arabidopsis. Plant Cell 24, 4850–4874. doi: 10.1105/tpc.112.107144
Rennenberg, H., Kreutzer, K., Papen, H., and Weber, P. (1998). Consequences of high loads of nitrogen for spruce (Picea abies) and beech (Fagus sylvatica) forests. New Phytol. 139, 1–86. doi: 10.1046/j.1469-8137.1998.00181.x
Roberson, E. D., and Mucke, L. (2006). 100 years and counting: prospects for defeating Alzheimer's disease. Science 314, 781–784. doi: 10.1126/science.1132813
Rosenthal, S. M., Fisher, E. R., and Stohlman, E. F. (1952). Nephrotoxic action of spermine. Proc. Soc. Exp. Biol. Med. 80, 432–434. doi: 10.3181/00379727-80-19647
Rosi, S., Ferguson, R., Fishman, K., Allen, A., Raber, J., and Fike, J. R. (2012). The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PLoS ONE 7:e31094. doi: 10.1371/journal.pone.0031094
Rubin, M. A., Berlese, D. B., Stiegemeier, J. A., Volkweis, M. A., Oliveira, D. M., dos Santos, T. L., et al. (2004). Mello CF Intra-amygdala administration of polyamines modulates fear conditioning in rats. J. Neurosci. 24, 2328–2334. doi: 10.1523/JNEUROSCI.1622-03.2004
Russell, D. H., and Snyder, S. H. (1968). Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl. Acad. Sci. U.S.A. 60, 1420–1427. doi: 10.1073/pnas.60.4.1420
Sacaan, A. I., and Johnson, K. M. (1990). Characterization of the stimulatory and inhibitory effects of polyamines on [3H]N-(1-[thienyl]cyclohexyl) piperidine binding to the N-methyl-d-aspartate receptor ionophore complex. Mol. Pharmacol. 37, 572–577.
Seiler, N., Sarhan, S., Graffel, C., Jones, R., Knodgen, B., and Moulinoux, J.-P. (1990). Endogenous and exogenous polyamines in support of tumor growth. Cancer Res. 50, 5077–5083.
Seiler, N., and Schmidt-Glenewinkel, T. (1975). Regional distribution of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J. Neurochem. 24, 791–795. doi: 10.1111/j.1471-4159.1975.tb03866.x
Shaw, K. N., Commins, S., and O'Mara, S. M. (2001). Lipopolysaccharide causes deficits in spatial learning in the water maze but not in BDNF expression in the rat dentate gyrus. Behav. Brain Res. 124, 47–54. doi: 10.1016/S0166-4328(01)00232-7
Shen, W., and Huber, S. C. (2006). Polycations globally enhance binding of 14-3-3 to target proteins in spinach leaves. Plant Cell Physiol. 47, 764–771. doi: 10.1093/pcp/pcj050
Shimada, A., London, E. D., Muhkin, A., Spangler, E. L., and Ingram, D. K. (1995). Polyamine modulation of NMDA receptors as a strategy for cognitive enhancement in aged rats. Soc. Neurosci. Abstr. 21:198.
Shukla, V., and Mattoo, A. K. (2013). “Developing robust crop plants for sustaining growth and yield under adverse climatic changes,” in Climate Change and Plant Abiotic Stress Tolerance, eds N. Tuteja and S. Gill (Weinheim: Wiley-VCH Verlag), 27–56
Sievert, H., Pällmann, N., Miller, K. K., Hermans-Borgmeyer, I., Venz, S., Sendoel, A., et al. (2014). A novel mouse model for inhibition of DOHH mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis. Model. Mech. 7, 963–976. doi: 10.1242/dmm.014449
Signor, C., Mello, C. F., Porto, G. P., Ribeiro, D. A., and Rubin, M. A. (2014). Spermidine improves fear memory persistence. Eur. J. Pharmacol. 730, 72–76. doi: 10.1016/j.ejphar.2014.02.035
Silva, C. M. G., and Gloria, M. B. A. (2002). Bioactive amines in chicken breast and thigh after slaughter and during storage at 4 ± 1°C and in chicken-based meat products. Food Chem. 78, 241–248. doi: 10.1016/S0308-8146(01)00404-6
Simon-Sarkadi, L., Holzapfel, W. L., and Halasz, A. (1994). Biogenic amine content and microbial contamination of leafy vegetables during storage at 50 C. J. Food Biochem. 17, 407–418. doi: 10.1111/j.1745-4514.1993.tb00483.x
Singh, A., Ansari, M. W., Raul, V., Singh, C. P., Shukla, A., Pant, R. C., et al. (2014). First evidence of putrescine involvement in mitigating the floral malformation in mangoes: a scanning electron microscopy study. Protoplasma 251, 1255–1261. doi: 10.1007/s00709-014-0611-6
Slocum, R. D., and Flores, H. E. (1991). Biochemistry and Physiology of Polyamines in Plants. Boca Raton, FL: CRC Press.
Sobieszczuk-Nowicka, E. (2017). Polyamine catabolism adds fuel to leaf senescence. Amino Acids 49, 49–56. doi: 10.1007/s00726-016-2377-y
Sobolev, A., Neelam, A., Fatima, T., Shukla, V., Handa, A. K., and Mattoo, A. K. (2014). Genetic introgression of ethylene-suppressed transgenic tomatoes with higher-polyamines trait overcomes many unintended effects due to reduced ethylene on the primary metabolome. Front. Plant Sci. 5:632. doi: 10.3389/fpls.2014.00632
Soda, K., Dobashi, Y., Kano, Y., Tsujinaka, S., and Konishi, F. (2009b). Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 44, 727–732. doi: 10.1016/j.exger.2009.08.013
Soda, K., Kano, Y., Sakuragi, M., Takao, K., Lefor, A., and Konishi, F. (2009a). Long-term oral polyamine intake increases blood polyamines concentrations. J. Nutr. Sci. Vitaminol. 55, 361–366. doi: 10.3177/jnsv.55.361
Soulet, D., and Rivest, S. (2003). Polyamines play a critical role in the control of the innate immune response in the mouse central nervous system. J. Cell Biol. 62, 257–268. doi: 10.1083/jcb.200301097
Sriva, A., Chung, S. H., Fatima, T., Datsenka, T., Handa, A. K., and Mattoo, A. K. (2007). Polyamines as anabolic growth regulators revealed by transcriptome analysis and metabolite profiles of tomato fruits engineered to accumulate spermidine and spermine. Plant Biotechnol. 24, 57–70. doi: 10.5511/plantbiotechnology.24.57
Srivenugopal, K. S., and Adiga, P. R. (1980). Coexistence of two pathways of spermidine biosynthesis in Lathyrus sativus seedlings. FEBS Lett. 112, 260–264. doi: 10.1016/0014-5793(80)80193-1
Tadano, T., Hozumi, S., Yamadera, F., Murata, A., Niijima, F., Tan-No, K., et al. (2004). Effects of NMDA receptor-related agonists on learning and memory impairment in olfactory bulbectomized mice. Methods Find Exp. Clin. Pharmacol. 26:93. doi: 10.1358/mf.2004.26.2.800060
Takano, A., Kakehi, J.-I., and Takahashi, T. (2012). Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol. 53, 606–616. doi: 10.1093/pcp/pcs019
Tang, W., and Newton, J. R. (2005). Polyamines promote root elongation and growth by increasing root cell division in regenerated Virginia pine. Plant Cell Rep. 24, 581–589. doi: 10.1007/s00299-005-0021-5
Tavernarakis, N. (2008). Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol. 18, 228–235 doi: 10.1016/j.tcb.2008.02.004
Tavladoraki, P., Cona, A., Federico, R., Tempera, G., Viceconte, N., Saccoccio, S., et al. (2012). Polyamine catabolism: target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 42, 411–426. doi: 10.1007/s00726-011-1012-1
Tiburcio, A. F., Altabella, T., Bitrián, M., and Alcázar, R. (2014). The roles of polyamines during the lifespan of plants: from development to stress. Planta 240, 1–18. doi: 10.1007/s00425-014-2055-9
Tiburcio, A. F., Kaur-Sawhney, R., and Galston, A. W. (1993). Spermidine biosynthesis as affected by osmotic stress in oat leaves. Plant Growth Regul. 13, 103–109. doi: 10.1007/BF00207599
Tisi, A., Angelini, R., and Cona, A. (2011a). Does polyamine catabolism influence root development and xylem differentiation under stress conditions? Plant Signal. Behav. 11, 1844–1847. doi: 10.4161/psb.6.11.17640
Tisi, A., Federico, R., Moreno, S., Lucretti, S., Moschou, P. N., Roubelakis-Angelakis, K. A., et al. (2011b). Perturbation of polyamine catabolism can strongly affect root development and xylem differentiation. Plant Physiol. 57, 200–215. doi: 10.1104/pp.111.173153
Tolbert, D. W., Ekstrom, J. L., Mathews, I. I., Secrist, J. A. III., Kapoor, P., Pegg, A. E., et al. (2001). The structural basis for substrate specificity and inhibition of human S-adenosylmethionine decarboxylase. Biochemistry 40, 9484–9494. doi: 10.1021/bi010735w
Tomitori, H., Usui, T. N., Saeki, N., Ueda, S., Kase, H., Nishimura, K., et al. (2005). Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke 36, 2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d
Uemura, T., Tachihara, K., Tomitori, H., Kashiwagi, K., and Igarashi, K. (2005). Characteristics of the polyamine transporter TPO1 and regulation of its activity and cellular localization by phosphorylation. J. Biol. Chem. 280, 9646–9652. doi: 10.1074/jbc.M410274200
Upp, J. R. Jr., Saydjari, R., Townsend, C. M. Jr., Singh, P., Barranco, S. C., and Thompson, J. C. (1988). Polyamine levels and gastrin receptors in colon cancers. Ann. Surg. 207, 662–669. doi: 10.1097/00000658-198806000-00004
Urano, K., Yoshiba, Y., Nanjo, T., Ito, T., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2004). Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem. Biophys. Res. Commun. 313, 369–375. doi: 10.1016/j.bbrc.2003.11.119
Veciana-Nogues, M. T., Marine-Font, A., and Vidal-Carou, M. C. (1997). Changes in biogenic amines during the storage of Mediterranean anchovies immersed in oil. J. Agric. Food Chem. 45, 1385–1389. doi: 10.1021/jf9605714
Velloso, N. A., Dalmolin, G. D., Gomes, G. M., Rubin, M. A., Canas, P. M., Cunha, R. A., et al. (2009). Spermine improves recognition memory deficit in a rodent model of Huntington's disease. Neurobiol. Learn. Mem. 92, 574–580. doi: 10.1016/j.nlm.2009.07.006
Wallace, H. M., and Caslake, R. (2001). Polyamines and colon cancer. Eur. J. Gastroenterol. Hepatol. 13, 1033–1039. doi: 10.1097/00042737-200109000-00006
Wallace, H. M., Fraser, A., and Hughes, A. (2003). A perspective of polyamine metabolism. Biochem. J. 376, 1–14. doi: 10.1042/bj20031327
Watson, M. B., Emory, K. K., Piatak, R. M., and Malmberg, R. L. (1998). Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana exhibit altered root growth. Plant J. 13, 231–239. doi: 10.1046/j.1365-313X.1998.00027.x
Wen, X. P., Pang, X. M., Matsuda, N., Kita, M., Inoue, M., Hao, Y. J., et al. (2008). Overexpression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res. 17, 251–263. doi: 10.1007/s11248-007-9098-7
Wi, S. J., Kim, W. T., and Park, K. Y. (2006). Over expression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rp. 25, 1111–1121. doi: 10.1007/s00299-006-0160-3
Williams, K. (1997). Interactions of polyamines with ion channels. Biochem. J. 325 289–297 doi: 10.1042/bj3260943
Williams, K., Romano, C., and Molinoff, P. B. (1989). Effects of polyamines on the binding of [3H]MK-801 to the N-methyl-D-aspartate receptor: pharmacological evidence for the existence of a polyamine recognition site. Mol. Pharmacol. 36, 575–581.
Williams, K., Zappia, A. M., Pritchett, D. B., Shen, Y. M., and Molinoff, P. B. (1994). Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol. Pharmacol. 45, 803–809.
Yamaguchi, K., Takahashi, Y., Berberich, T., Imai, A., Miyazaki, A., Takahashi, T., et al. (2006). The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett. 580, 6783–6788. doi: 10.1016/j.febslet.2006.10.078
Yamaguchi, O., and Otsu, K. (2012). Role of autophagy in aging. J. Cardiovasc. Pharmacol. 60, 242–247. doi: 10.1097/FJC.0b013e31824cc31c
Yano, Y., Kataho, N., Watanabe, M., Nakamura, T., and Asano, Y. (1995). Changes in the concentration of biogenic amines and application of tyramine sensor during storage of beef. Food Chem. 54, 155–159. doi: 10.1016/0308-8146(94)00161-W
Yatin, S. M., Yatin, M., Varadarajan, S., Ain, K. B., and Butterfield, D. A. (2001). Role of spermine in amyloid beta-peptide-associated free radical-induced neurotoxicity. J. Neurosci. Res. 63, 395–401. doi: 10.1002/1097-4547(20010301)63:5<395::AID-JNR1034>3.0.CO;2-Q
Yoshimoto, K., Takamura, H., Kadota, I., Motose, H., and Takahashi, T. (2016). Chemical control of xylem differentiation by thermospermine, xylemin, and auxin. Sci. Rep. 6:21487. doi: 10.1038/srep21487
Zabala-Letona, A., Arruabarrena-Aristorena, A., Martín-Martín, N., Fernandez-Ruiz, S., Sutherland, J. D., Clasquin, M., et al. (2017). mTORC1-dependent AMDI regulation sustains polyamine metabolism in prostrate cancer. Nature 547, 109–113. doi: 10.1038/nature22964
Ziegler, W., Hahn, M., and Wallnöfer, P. R. (1994). Changes in biogenic amine contents during processing of several plant foods. Deutsche Lebensmittel Rundschau. 90, 108–112.
Keywords: biogenic amines, nutrition, metabolism, polyamines and human diseases, vegetables, fruits, cereals, meats
Citation: Handa AK, Fatima T and Mattoo AK (2018) Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 6:10. doi: 10.3389/fchem.2018.00010
Received: 13 September 2017; Accepted: 15 January 2018;
Published: 05 February 2018.
Edited by:
Dominique Van Der Straeten, Ghent University, BelgiumReviewed by:
Taku Takahashi, Okayama University, JapanVasileios Fotopoulos, Cyprus University of Technology, Cyprus
Copyright © 2018 Handa, Fatima and Mattoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Avtar K. Handa, ahanda@purdue.edu
Autar K. Mattoo, autar.mattoo@ars.usda.gov
 Avtar K. Handa
Avtar K. Handa Tahira Fatima
Tahira Fatima Autar K. Mattoo
Autar K. Mattoo