Acute Drought Is an Important Driver of Bark Beetle Infestation in Austrian Norway Spruce Stands
- 1Department of Forest and Soil Sciences, Institute of Forest Entomology, Forest Pathology and Forest Protection, University of Natural Resources and Life Sciences, BOKU, Vienna, Austria
- 2Independent Researcher, Munich, Germany
- 3Department of Forest and Soil Sciences, Institute of Forest Ecology, University of Natural Resources and Life Sciences, BOKU, Vienna, Austria
Infestations by the Eurasian spruce bark beetle, Ips typographus have recently caused peaks in Norway spruce mortality in Central European forests. In this study, we examined how temperature conditions, chronic and acute drought stress, and stand characteristics influence forest disturbance by the spruce bark beetle. We investigated bark beetle induced salvage logging in Norway spruce stands of Austrian Federal Forests (ÖBf) as a proxy variable for infestation/attack by I. typographus. Utilizing ÖBf forest inventory data and the monitoring tool PHENIPS-TDEF, a well-proven bark beetle phenology model combined with a forest water balance module, we retrospectively simulated effective temperature sums for bark beetle development and transpiration deficits at forest stand level. We examined forest stand properties and the model output variables as predictors of bark beetle attack in decision trees and binary logistic regression analysis. We found that I. typographus infestation increased with a stand predisposition index indicating high share of Norway spruce, increased stand age, and stand density. Stands subject to bark beetle attack in the previous year were highly prone to subsequent damage, which points to attack pressure from increased population densities due to ample supply of breeding material. While chronically dry soil conditions described as shallow, xeric, and of low moisture, were associated with bark beetle infestation to a lesser degree, acute drought in the form of increases in stand transpiration deficits proved to raise the probability of bark beetle attacks. The previous year's and current year's summer (June to August) TDEF total, in combination with effective thermal sums allowing for at least two bark beetle generations and sister broods, were significant predictors of bark beetle attack. We conclude from our results that in the absence of windthrow, a combination of ample host availability, favorable temperature conditions for bark beetle development, and acute disposition of trees to attack caused by drought stress can intensify population growth and very likely lead to bark beetle mass outbreaks.
Introduction
European conifer forests have been subject to frequent and severe abiotic and biotic damage events, a pattern which has been likely driven on the one hand by high disturbance susceptibility due to stand history, forest development stage, and structure (Schelhaas et al., 2003; Schurman et al., 2018), and on the other hand by climate change (Seidl et al., 2015). Extreme weather conditions such as prolonged drought and high wind speeds have co-occurred with peaks in forest mortality in Central European forest landscapes over the past two decades (Senf and Seidl, 2018). The most important biotic disturbance agent of Norway spruce (Picea abies; Pinales: Pinaceae), the Eurasian spruce bark beetle Ips typographus (Coleoptera: Curculionidae), has profited from rising spring and summer temperatures, precipitation deficits and extensive breeding material provided by storm damage (Jönsson et al., 2009; Marini et al., 2013; Netherer et al., 2015). The amount of timber salvaged due to attacks of the Eurasian spruce bark beetle increased after the years 1990 and 2000 in most European countries. The increases in forest damage observed in multi-year records by Marini et al. (2017) were strongly related to climate stressors boosting bark beetle population densities even in the absence of storms. However, the relative importance of specific bark beetle disturbance drivers presumably shifts with environmental conditions depending on geographic location, elevation, and topography of forest sites (Temperli et al., 2013). Bark beetle-induced forest mortality is, hence, expected to be unevenly distributed over heterogeneous forest landscapes such as in Austria, with presumable differences in magnitude between lowland and High Mountain/alpine Norway spruce stands.
Models to simulate bark beetle population dynamics offer the opportunity to predict outbreak hazards and variations in spatial and temporal attack patterns (Logan et al., 1998; Negrón et al., 2008; Coops et al., 2012). Infestations by comparably “aggressive” Ips and Dendroctonus species in a similar manner depend on site and stand related characteristics including tree species composition, host tree volume, stand age structure and density, site productivity, topographical features, temperature, and precipitation (Netherer and Nopp-Mayr, 2005; Dymond et al., 2006; Fettig et al., 2007; Stadelmann et al., 2013). Availability of breeding resources, the most important factor influencing population growth and size, strongly depends on recent abiotic disturbance history, implemented forest management practices, and spatial spreading of attack spots (Okland and Bjørnstad, 2006; Pasztor et al., 2014). The majority of new infestations occur within a radius of 100–500 m from attacked trees, and epidemic conditions going along with high breeding densities even promote short-distance dispersal behavior of I. typographus (Kautz et al., 2011). Salvage cuttings in managed forests can therefore be effective only when performed before offspring emergence and in close vicinity of storm damages and acute infestation spots (Stadelmann et al., 2014). Increased dimensions of salvaged timber indicate both, high attack pressure and colonization densities in preceding years as well as alert forest protection. Pest management should consider population dynamics and predisposition of forest stands to various disturbance agents. The most significant predictors of bark beetle disturbance thus need to be identified and integrated into a universal model framework (Hanewinkel et al., 2010; Seidl et al., 2011). A reliable evaluation of bark beetle outbreak risks on landscape scale can be based on key variables obtained from easily accessible forest data sets in combination with the simulation of insect phenology and tree mortality as demonstrated for D. ponderosae infestations (Robertson et al., 2008; Safranyik et al., 2012).
Ips typographus phenology has been comprehensively investigated with particular regard to the influence of temperature on flight and brood development (Annila, 1969; Lobinger, 1994; Wermelinger and Seifert, 1998, 1999), which has facilitated a robust temperature-based simulation of this bark beetle's life cycle. A warming climate speeds up spring swarming and development from egg to adult, thereby consolidating viable populations even at high elevations and latitudes (Lange et al., 2006; Jönsson et al., 2009). Accelerated generation development in response to above-average temperature conditions further driven by prolonged late summer periods might result in the common establishment of two bark beetle generations in southern Fennoscandia/three generations in lowland areas of Central Europe in the near future (Jönsson et al., 2011; Temperli et al., 2013). The phenological model PHENIPS (Baier et al., 2007) is suited for a refined prediction of bark beetle development at stand/tree level by explicitly considering the strong effects of regional topography as well as stand density on local air and bark temperature. Using records from a local weather station or regionalized climate data, the model simulates the seasonal maximum/minimum number of I. typographus generations by calculating the effective degree-days available for seasonal bark beetle development. PHENIPS, which was repeatedly implemented and validated in bark beetle risk studies (Seidl et al., 2007; Hlasny et al., 2011; Berec et al., 2013; Stadelmann et al., 2013; Temperli et al., 2013; Pasztor et al., 2014; Mezei et al., 2017) remains by now the only phenology model operating as a real-time monitoring instrument in Europe1. However, the ultimate understanding of successful/failed spruce bark beetle attack and brood establishment is still confined by knowledge gaps concerning the effects of environmental stress on Norway spruce susceptibility. Recent simulation approaches such as the ecosystem model LPJ-GUESS by Jönsson and Lagergren (2018) attempt to include effects of different climate scenarios and varying bioclimatic zones and soil conditions in modeling forest drought stress and predisposition to biotic disturbance. The model builds on the hypothesis that a continuous depletion of non-structural carbohydrate stores as a consequence of drought episodes is diminishing the availability of secondary metabolites for tree resistance mechanisms and disposing Norway spruce to bark beetle attack (Bréda et al., 2006). Yet, the challenge remains for such risk assessment systems to translate the occurrence of heat and drought events into meaningful tree stress proxies and infestation probabilities.
Precipitation deficits interpreted as drought have repeatedly proved significant in explaining timber volumes salvaged due to I. typographus attack (Faccoli, 2009; Marini et al., 2013). Expanding the definition of drought into a function of precipitation, temperature, and soil water-holding capacity can enhance the simulation of forest dynamics and biotic disturbance under climate change scenarios (Seidl et al., 2007; Temperli et al., 2013). Such ecosystem modeling approaches have, however, rarely been linked to site conditions and tree/bark beetle interactions actually investigated at local level and, furthermore, have yet to be verified. To address this problem, seasonal variations in the stress status of mature Norway spruce and shifting capability to defend I. typographus attacks were studied in the course of an elaborate drought manipulation experiment performed in a forest stand located in the Eastern Austrian Rosalia Mountains (Netherer et al., 2015, 2016). The “Rosalia Roof experiment” for the first time provided strong empirical evidence of the negative impact of drought stress on Norway spruce defense against I. typographus; yet, distinct differences in attractiveness of stressed and control trees were not observed (Netherer et al., 2015; Matthews et al., 2018). Notably, proportions of prevented attacks were higher among trees exhibiting low water stress and high resin flow, while proportions of successful attacks increased with drought stress (Netherer et al., 2015). Using data from the study site of the “Rosalia Roof experiment,” a process-based water balance model (TDEF), simplified for the purpose of practical application, was parametrized to simulate stand hydrology. Acute and chronic transpiration deficits (i.e., differences between potential evapotranspiration simulated under non-limiting soil moisture and actual evapotranspiration), calculated as proxies for Norway spruce drought stress status, in part explained I. typographus attack success observed in the bioassays performed at the study trees (Matthews et al., 2018). These results were thus behind the rationale for combining the PHENIPS and TDEF models into a single tool, PHENIPS-TDEF, which by simulating both bark beetle phenology and stand hydrology can identify critical periods of coinciding I. typographus flight and Norway spruce drought stress (Netherer et al., 2019).
In this study, we test for the first time the utility of this tool for predicting stand-level bark beetle infestation risk. We hypothesize a non-linear relationship between host susceptibility and drought, with successful bark beetle attack of trees depending on the timing and intensity of stress events, and whether water deficiencies are acute or chronic. In this study we utilize the comprehensive inventory dataset of the Austrian Federal Forests (ÖBf) AG, which allowed us to study important drivers of recent bark beetle disturbance events with focus on predisposing stand characteristics, temperature dependent bark beetle phenology, and chronic as well acute drought episodes. Our main goals were to (1) investigate whether frequencies of bark beetle attack in Norway spruce stands differ with water supply influenced by local site conditions and stand composition and structure (stand predisposition index) as documented in the forest inventory database; (2) parametrize the water balance module TDEF with regard to the various site and stand conditions present in the ÖBf forest enterprises and retrospectively simulate transpiration deficits accumulated in specific observation periods; and (3) examine to what extent stand structural features, temperature dependent potential bark beetle development modeled by PHENIPS, transpiration deficits, and harvesting activities in the preceding year can explain the presence/absence of bark beetle attack in management units of ÖBf forest enterprises.
Materials and Methods
This study was based on data related to forest stand and soil conditions, weather, and bark beetle induced salvage logging records (Table 1). The variable set for statistical analyses was either calculated from basic data directly or indirectly, by use of the phenological model PHENIPS and the TDEF module.
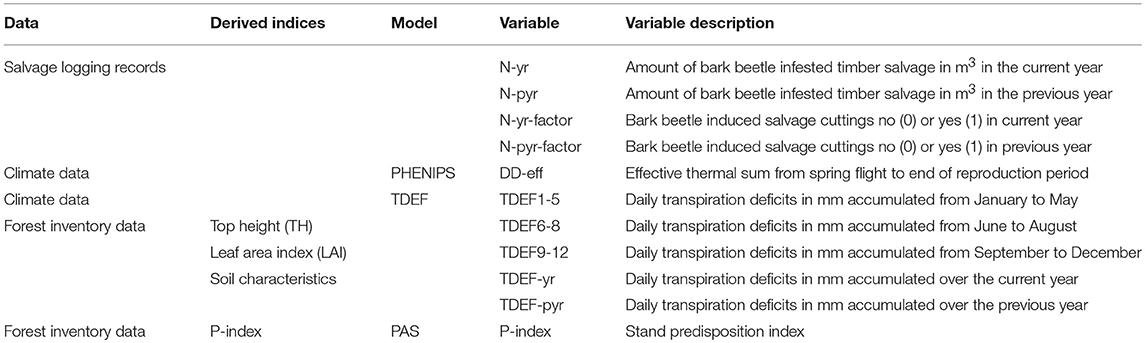
Table 1. Used database, parameters derived from these data as input parameters for the models PHENIPS and TDEF, and variables derived either directly from database or by use of the models.
Study Area and Database
Forest Inventory (FI) Dataset
The data analyzed here was provided by the Austrian Federal Forests (ÖBf) which manage 15% of the Austrian forested area. The ÖBf forest inventory (FI) dataset, the last time revised in 2010, is split to 12 independent forest enterprises that cover 510,000 ha of forests distributed over alpine, mountainous, and lowland areas (Figure 1). Table 2 lists the enterprises with their respective abbreviations used in this publication. The smallest management units within forest districts of each ÖBf forest enterprise are sub-divisions, which represent forest stands. For each of the 81,446 forest stands where Norway spruce was present (331,291 ha), we obtained information on the proportion of tree species (0–100%), tree age (40–400 years for Norway spruce), stand density index, and site index (productivity class). Furthermore, an informal description of site characteristics including geology, vegetation and water supply included in the FI database was referred to in this study.
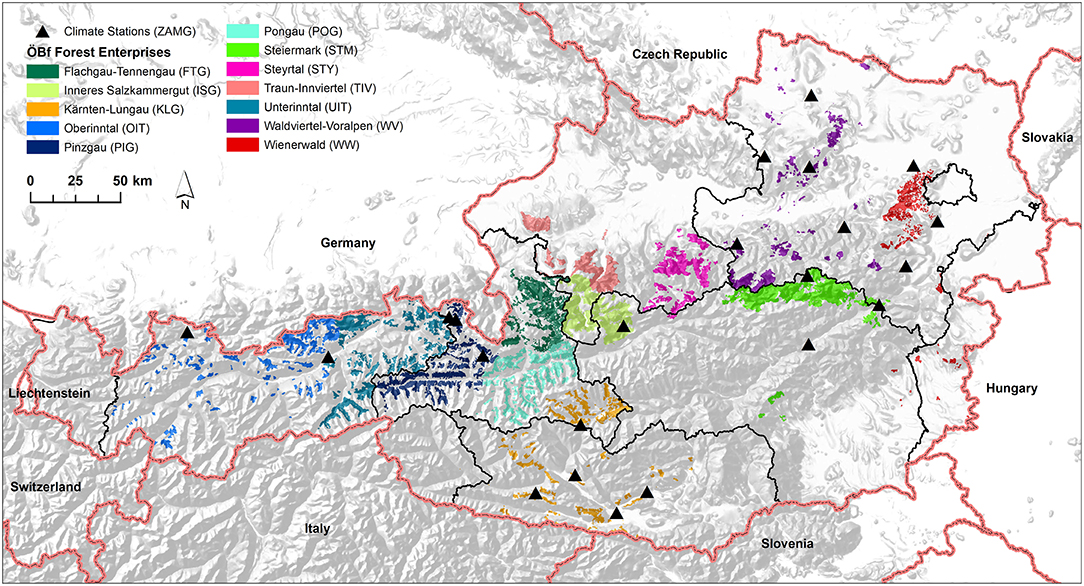
Figure 1. Map showing the geographical distribution of Austrian Federal Forests (ÖBf) forest enterprises (12) and weather stations of the Austrian institution for meteorology and geodynamics (ZAMG) (22).
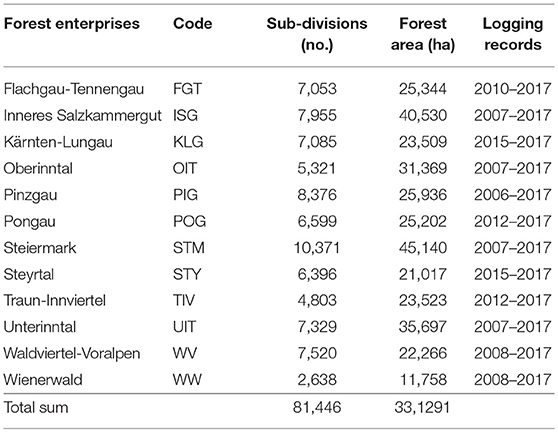
Table 2. German names and codes of ÖBf forest enterprises, number of sub-divisions (forest stands), and corresponding forest area examined, and period for which logging records were available.
Salvage Logging Due to Bark Beetle Infestation
We accessed yearly records of salvaged timber in cubic meters (m3) due to bark beetle infestation on ÖBf forest stand level. ÖBf forest enterprises are legally obliged to remove attacked trees and have systematically recorded salvage loggings with regard to even small volumes, dividing bark beetle infested timber from harvests due to other causes (e.g., storm felled trees). Salvage logging datasets relating to month of measurement, and type of harvest (pre- or final harvest) went back 10 years for some forest enterprises but covered 3 years at least (Table 2). Under consideration of the assumed time lag between initial bark beetle attack of stands, detection of infested trees by the foresters, eventual timber harvest and measurements, harvesting activities from January to June in fact belong to the previous year's bark beetle infestation (N-pyr), from July to December to current year's attack (N-yr). As ÖBf stands strongly vary in size (less than one to several hectares), the total amount of bark beetle infested timber in m3 is an inappropriate proxy of attack intensity. We thus considered whether a forest stand was infested at all, and therefore transformed damage data into binary variables (N-factor, N-pyr-factor) describing absence/presence of attack (Table 1).
Climate Data
The Austrian institution for meteorology and geodynamics2 provided climate data of 22 weather stations (Figure 1). For each forest district, the nearest climate station within a maximum distance of 50 km was assigned, using records of temperature, precipitation, relative humidity, wind as well as global radiation for the period 2006–2017. For each forest stand, hourly temperature was adjusted by moist adiabatic lapse rate using 5°C/km (Dodson and Marks, 1997). Hourly global radiation was adapted by means of potential global radiation (Baier et al., 2007) to account for the effects of orographic heterogeneous terrain.
Simulation of Bark Beetle Phenology and Stand Transpiration Deficits by PHENIPS-TDEF
The dual bark beetle phenology-forest water balance simulator PHENIPS-TDEF was developed to help identification of potentially co-occurring beetle flight and tree drought stress in forest stands (Matthews et al., 2018). The PHENIPS tool was originally described by Baier et al. (2007) and can be easily implemented following this publication. Here, we used the model for a direct translation of weather conditions into the potential number of fully developed bark beetle generations per observation year. We retrospectively summed up seasonal effective thermal sums, i.e., degree days (dd) above the developmental threshold for generation development of Ips typographus (i.e., 8.3°C) after the onset of spring flight and first attack of trees, and we furthermore considered sister broods that are produced in the course of a repeated attack of female parental beetles. Simulations were based on daily mean and maximum air temperature and mean global radiation on forest stand level. For the interpretation of temperature thresholds in the section Classification Trees it is important to know that complete development of the first filial generation requires a minimum effective thermal sum (DD-eff, Table 1) of 557 dd (Baier et al., 2007). Thermal sums, DD-eff > 2,228 dd accumulated at forest stand level correspond to more than two filial generations and two sister broods, which potentially results in increased reproduction rates of bark beetle populations.
The module TDEF indicates drought stress of potential bark beetle host trees by calculating a transpiration deficit (difference between potential and actual transpiration). The basic ecosystem water balance model was written and run using the open source programming environment R (R Core Team, 2014). As described in detail by Matthews et al. (2018), TDEF transforms the hourly weather data depending on inter alia the elevation of the weather station and the forest stand to be simulated, and the slope and aspect of the stand. The calculation starts when the snow-free period, estimated for each year as the first of day of 5 consecutive days when the daily mean temperature exceeds 5°C, begins. Simulations follow a two-bucket approach, with one reservoir representing the soil water storage in the rooting zone, the other reservoir the interception of water by the forest canopy. Initially, soil volumetric water content at field capacity is solved by the Brooks-Corey equation (Brooks and Corey, 1964) and is multiplied by the rooting depth to give the starting total soil water storage in mm. Required input parameters (Table 3) are the saturated water content (SWC), the residual water content (RWC), the air entry pressure head (α), and the unimodal pore size distribution (λ). These variables can be determined from soil physical properties using pedo-transfer functions (comp. section Soil Characteristics). The derived SWC is reduced with increasing volumetric stone content of the rooting depth. Consecutive simulation steps comprise hourly calculations of the radiation balance at the forest floor and canopy, and time-series of potential evaporation of intercepted water by solving a form of the Penman-Monteith equation for potential evaporation (Monteith and Unsworth, 2008). Interception and throughfall are inter alia influenced by the canopy's dryness, the maximum amount of intercepted water the canopy can store, and the proportion of forest floor covered by the canopy calculated as functions of the leaf area index (LAI). Iteratively, hourly potential and actual transpiration, soil evapotranspiration, and soil water storage is simulated. The final output is a time-series of daily transpiration deficits (TDEF) per forest stand, which were aggregated to variables representing drought stress of stands in particular periods (Table 1).
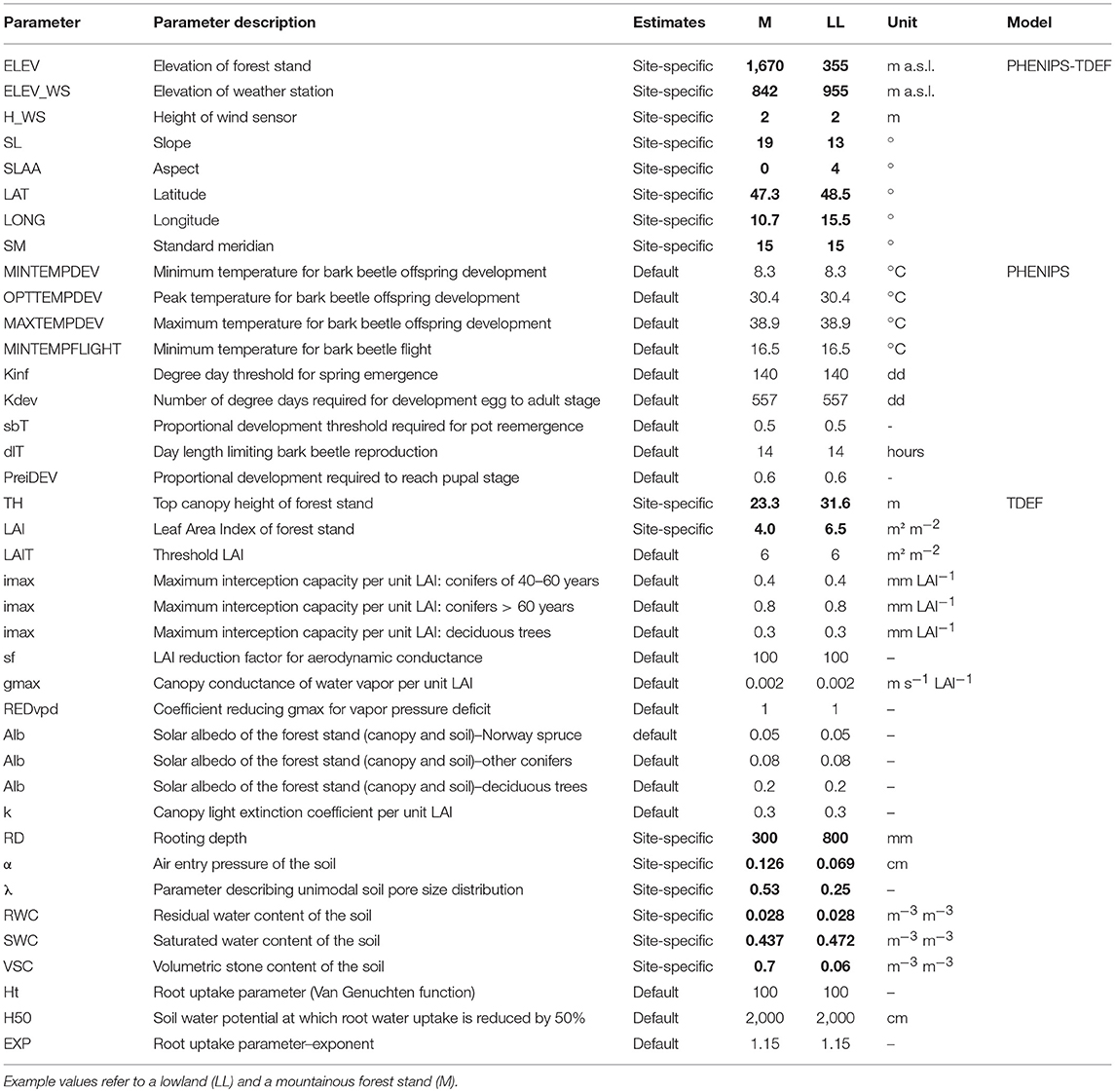
Table 3. Default and site specific (bold) input parameters for PHENIPS-TDEF simulations according to the overview of Matthews et al. (2018).
Calculation of Forest Stand Related Indices
The input parameters required by PHENIPS-TDEF are listed in Table 3. The necessary forest stand related indices, top height and leaf area index, cannot be directly obtained from the FI dataset and have to be calculated from functional relationships. Stand predisposition index was derived in order to test for the influence of stand characteristics on salvage cuttings due to bark beetle attack.
Top Height of Norway Spruce (TH)
Top height of Norway spruce is a variable in the water balance module TDEF necessary to simulate aerodynamic resistance to water vapor exchange at the canopy. Given the site index, and stand age as defined in the FI dataset, the parameter was calculated for each forest stand based on the yield functions published by Marschall (1988). The equation is as follows:
where i indicate a specific site index class, j a specific Norway spruce stand, and β is a constant derived from the biometric relationships within a specific growth area.
Leaf Area Index (LAI)
Leaf area significantly influences interception and transpiration dynamics in the canopy (Köstner et al., 2002). As an exact determination by sampling of twigs, use of hemispherical photography or plant canopy analyzers (Gower and Norman, 1991) for all the simulated stands was not possible, proxy variables had to be used instead, e.g., derived from forest crown measures (Laubhann et al., 2010). Here, we estimated canopy variables based on the relationship between tree/stand age (years) and leaf area index (m2/m2) as described by Yu et al. (2014) for Norway spruce monoculture stands in the western Czech Republic. We fitted the function to an expanded dataset which included data used to fit the original Yu et al. (2014) model as well as LAI records for European Norway spruce stands of different stand age listed in the “LAI_Woody_Plants” dataset3 of Nasa Earth Data (Iio and Ito, 2014).
According to Yu et al. (2014), the LAI of Norway spruce stands can be described by a second-degree polynomic function:
where j indicates a specific Norway spruce stand.
The fitted function includes the following parameter values to calculate LAI: α = 4.21, β1 = 9.85 × 10−2, β2 = 6.0 × 10−4 (n = 100; R2 = 0.355; Figure S1a). The measures derived from this curve increase with stand age up to 80 years with a maximum value of 8.25, then decrease again to reach negative values for spruce stands ≥200 years. Due to a lack of empirical data for Norway spruce stands older than 160 years necessary for a better fit of the function, and excluding significant needle losses, the LAI of four at 165 years stand age was assumed also for stands aged above 165 years. Furthermore, variation in LAI records from the Nasa Earth dataset was particularly high for spruce stands ≤ 40 years, an age class that was however excluded from the ÖBf FI for the present study. We calculated LAI for each forest stand, also taking into account admixture of tree species other than Norway spruce. Relationships between conifer and deciduous tree stand age and LAI were summarized by Iio and Ito (2014) and Breuer et al. (2003). To take the other main tree species into account, we used allometric functions for Pinus sylvestris, and the deciduous tree species beech (Fagus sylvatica) and oak (Quercus spp.) (Figures S1b,c). LAI values of deciduous stands however only slightly change with age but reach maxima in early summer.
Stand Predisposition Index (P-Index)
We calculated a simple index based on the proportion of Norway spruce, stand age, and stand density to evaluate stand related predisposition (P-index) to I. typographus. Relative weighting of the indicators and index calculation follows the concept of PAS presented by Netherer and Nopp-Mayr (2005). When applying the assessment system to FI data, the relative indicator weightings for each of the included parameters, e.g., proportion of Norway spruce, are aggregated (Table 4). The resulting sum is related to the maximum value achievable within the system, and the final index is interpreted as the relative predisposition level of the assessment unit. Generally, probability/hazard of I. typographus infestation increase with the dominance of Norway spruce and stand age (Wermelinger, 2004). In case Norway spruce was present in more than one stand layer, we derived indices for each layer and considered the maximum values for statistical analyses.
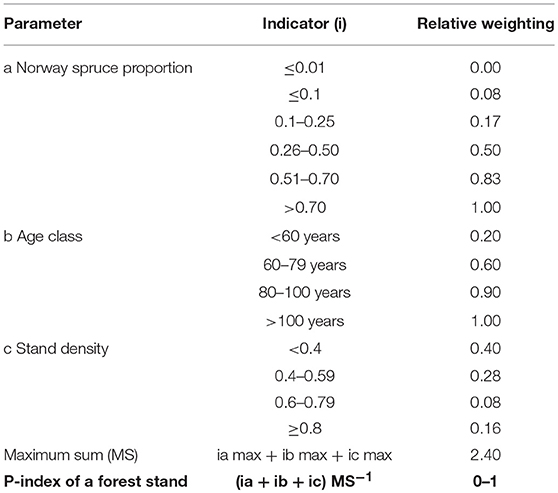
Table 4. Scheme for the calculation of the stand predisposition index (P-index) following the PAS approach of Netherer and Nopp-Mayr (2005) to relate accumulated stand-specific relative indicator weightings for parameters a-c to the maximum sum (MS) achievable within the assessment system.
Soil Characteristics
The water balance module TDEF requires input data that describe the physical properties of the soil with regard to water storage capacity (Table 3). While the FI dataset of ÖBf includes a site description related to each forest stand with specific regard to geology (flysch, limestone, silicate, sediment), water supply (five categories from xeric to water-logged), and soil depth (five categories from very low to high), information on soil texture and stone content is only qualitative. To provide an example, WW “steep and rocky, xeric, shallow, siliceous sites” typically describes forest stands at extreme locations in the mountainous and alpine area. The FI database describes 19.2% of all forest stands as shallow, xeric or of low moisture soil condition. We categorized xeric and low moisture sites with shallow soils as chronically dry, and moist sites with deeper soils as well water-supplied forest stands. Note that these static descriptions refer to water supply from the potential soil water storage point of view and are independent from precipitation. The key soil characteristics necessary for modeling, i.e., volumetric stone content (VSC), rooting depth (RD), soil texture class for SWC, RWC, α, and λ, were derived from qualitative FI information by expert interpretation and following the manual for soil mapping of the Austrian Research Center for Forests (BFW, 2014). We assigned soils described in the FI database as skeleton-rich a mean proportion of stones in the soil layer of 70%, i.e., VSC of 0.7. Soils of increased, moderate and low skeleton were interpreted to hold 45, 20, and 6% stones per soil depth, respectively. RD ranged from 300 mm for soils described as shallow in the FI dataset to 900 mm assumed for deep-rooted sites. Each of the 98 different categories of site description in the ÖBf FI dataset relating to the soil texture classes defined for Austrian soil mapping (BFW, 2014) were assigned to one of the main soil texture classes sand, silt, loam, and clay according to the USDA textural classification triangle (Soil Survey Division Staff, 1993). Under consideration of the differences between the two classification systems regarding particle size, we averaged estimates from the pedo-transfer functions presented by Rawls and Brakensiek (1989), which are listed in Table 5.
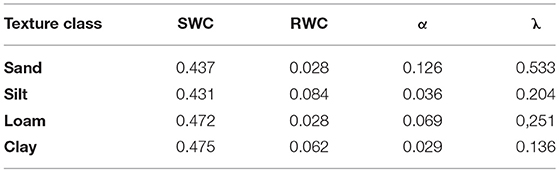
Table 5. Estimates for saturated water content (SWC), relative water content (RWC), air entry pressure of the soil (α), and unimodal soil pore size distribution (λ) from pedo-transfer functions of Rawls and Brakensiek (1989) used in TDEF.
Statistical Analyses
Based on the full dataset of 81,446 records (stands of all 12 forest enterprises) we used Pearson's Chi-squared test to examine the null hypothesis of an even distribution of salvage cuttings due to I. typographus attacks (bark beetle attacks in the following) among forest stands of differently high P-index as well as between chronically dry and well water-supplied forest stands. We used classification trees and binary logistic regression to analyze separate datasets of 10 ÖBf forest enterprises (except for FGT and KLG) including seven forest stand-, temperature-, and drought-related parameters (Table 1); and a merged dataset (forest stands of nine ÖBf forest enterprises except for FGT, KLG, and STY) regarding bark beetle attacks from 2014 to 2016.
Classification Trees
Using presence/absence of bark beetle salvage loggings as the grouping variable (N-yr), we analyzed the hierarchical interactions of the explanatory parameters in classification trees (CTs). We built CTs based on the Exhaustive CHAID (Chi-squared Automatic Interaction Detection) algorithm (IBM SPSS Statistics 21), which at each step chooses the independent predictor variable that has the strongest interaction with the dependent variable. The algorithm examines all possible splits for each predictor and merges categories in case they are not significantly different with respect to the dependent variable. We determined a maximum tree depth of three, and a minimum number of cases in super- and subordinate knots of 1,000 and 500, respectively. Higher misclassification costs of two were fixed for the case that presence of bark beetle attacks was incorrectly classified as absence in the model. We chose 10-fold cross-validation to test how well tree structure generalizes to a larger dataset. Accuracy of classification was measured by percentage of correct classifications, percentage of correct classification of attack, and the area under the receiver operating characteristic curve (AUC). An AUC of 0.5 stands for random models, while higher values indicated classification trees that predict correctly both, absence and presence of attack. AUC of 0.7 indicates good classification, >0.9 excellent classification.
Binary Logistic Regression
To analyze the effect of environment on bark beetle infestation, we developed generalized linear models (GLM) and specified a binomial error distribution and a logit-link function (Hosmer and Lemeshow, 2000). Continuous explanatory variables were centered (mean subtraction) and scaled (division by standard deviation). Model simplification was carried out using an automated stepwise backward model selection procedure based on the Akaike information criterium (AIC) using the function “stepAIC” from the R package “MASS” (v. 7.3–50, Venables and Ripley, 2002). As a measure of model calibration and refinement, we used R (Nagelkerke, 1991) which allows to distinguish between observed and predicted values and ranges between zero and one. To further evaluate the model fit, scaled residuals simulated from fitted models using the DHARMa R package (v. 2.0.2, Hartig, 2018) were calculated. We visualized the generated predictions of new bark beetle incidences using wireframe surface plots which were rendered using the R-package “lattice” (v. 0.20–35). This graphical representation allows to visual the combined effect of multiple predictors (everything else hold constant) on the response variable and is commonly used in ecology (Panassiti et al., 2013).
Results
Incidence of Bark Beetle Attack Depending on Site Water Supply and P-Index
Yearly amounts of salvaged timber followed different trends in the various ÖBf forest enterprises, but clearly increased since 2015 (Figure 2), when 12.7% of all Norway spruce stands were affected by bark beetles. In the forest enterprises TIV and WV, a maximum of 40.8 and 29% of forest stands, respectively, were infested in 2015. Proportions of attacked stands on the other hand remained low in western- and easternmost forest districts (UIT and OIT, 5.6%; WW, 6.8%). While almost 20% of all forest stands are described in the FI database as shallow, xeric or of low moisture soil condition, the share of such chronically dry sites lies above average in high mountain and alpine regions (e.g., STM, 28.7%; POG, 26.5%), and below average for enterprises which cover lowland areas to a large part (e.g., STY, 6.5%; TIV, 8.7%). Forest stands growing on well water-supplied sites were more often subject to bark beetle attack in 2015 and 2016 than chronically dry stands. This pattern was obvious across all forest enterprises (Chi-squared = 567.96, df = 1, p < 0.001), but most significant in forest enterprises showing highest amounts of bark beetle induced salvage loggings (TIV, Chi-squared = 185.28, df = 1, p < 0.001; WV, Chi-squared = 159.78, df = 1, p < 0.001) (Figures 3A–C).
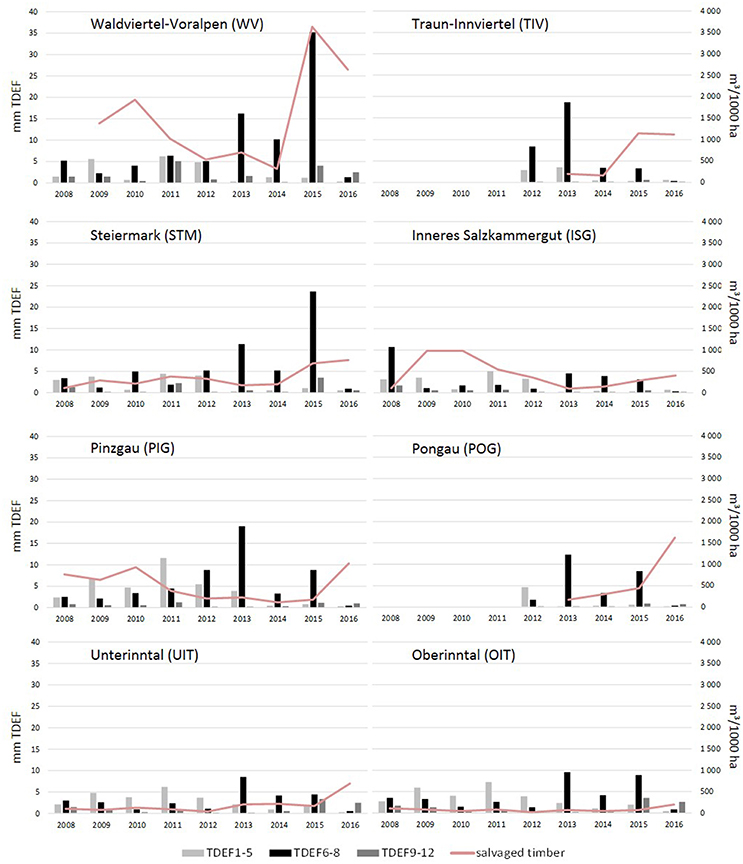
Figure 2. Drought stress and bark beetle attack in eight Austrian Federal Forests (ÖBf) forest enterprises (2008–2016): mean transpiration deficits in mm per periods January–May (TDEF1-5), June–August (TDEF6-8), September–December (TDEF9-12), and amount of timber salvaged due to bark beetle attack in m3/1,000 ha Norway spruce stands examined.
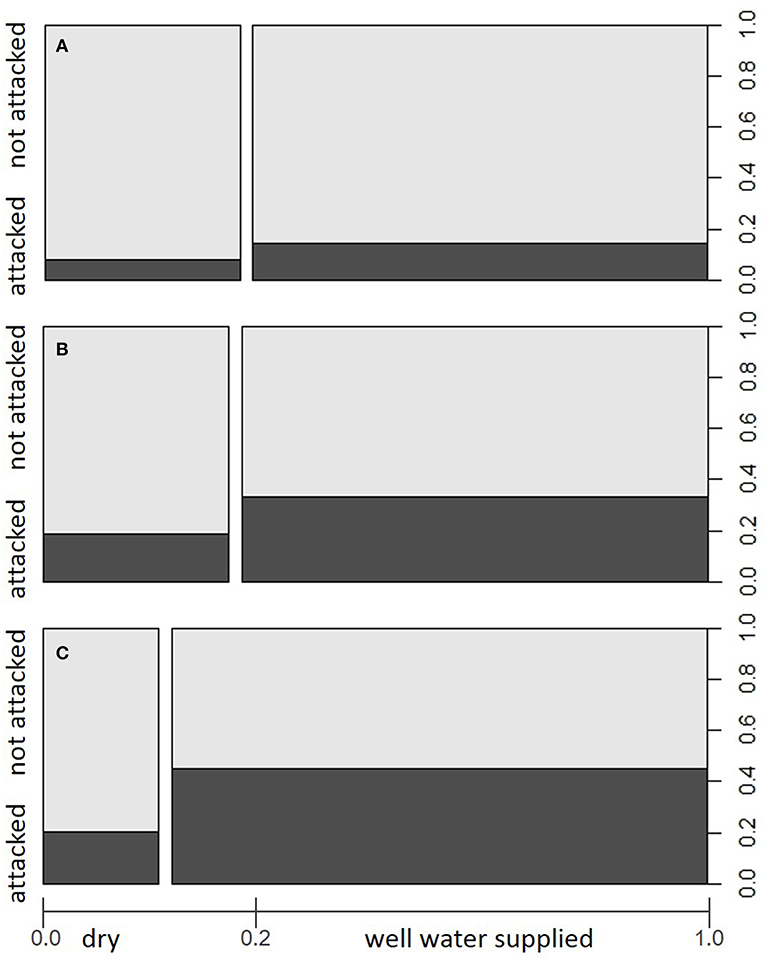
Figure 3. Proportions of forest stands attacked/not attacked by I. typographus in the period 2015–2016 within site categories dry and better water supplied with regard to (A) total study area (i.e., all examined Austrian Federal Forests (ÖBf) forest enterprises), (B) forest enterprise WV, and (C) forest enterprise TIV. The width of bars along the x-axis represents the relative share of site categories among examined forest stands.
Although we focused this study exclusively on forest stands where Norway spruce was present, stand predisposition patterns with regard to share of tree species, stand age, and stand density were not entirely homogeneous over the study area (comp. Supplementary Material, Figure S2). We found clearly right-skewed distributions toward stand predisposition index (P-index) classes in the range of 0.58–0.78 and 0.79–1.0, representing dense Norway spruce dominated stands of increased age in the enterprises located in High Mountain and alpine forest areas. Yet, comparably higher proportions of low to moderate predisposition categories (P-indices ranging between 0.15–0.36 and 0.37–0.57, respectively) documented the increased presence of tree species other than Norway spruce and lower age classes in forest stands of WW, WV, STY, and TIV. Apart from these stand structural differences between forest enterprises, the observed proportions of attacked forest stands within predisposition classes overall differed significantly from an even distribution (Chi-squared = 590, df = 3, p < 0.001). The relative increase in infested forest stands from 7.4% <8.9% <12.5% <15.5% in the categories of low < moderate < high < very high stand predisposition (Figure 4) underlines the significance of P-index in the variable set.
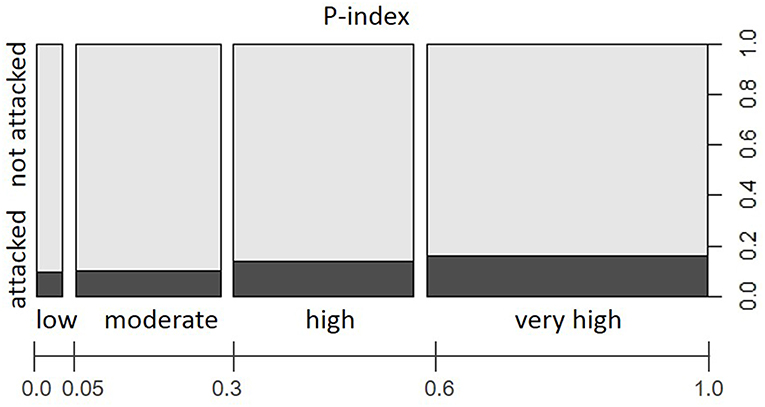
Figure 4. Proportions of forest stands with/without salvage loggings due to I. typographus attacks in the period 2015–2016 within P-index categories “low,” “moderate,” “high,” and “very high” with regard to total study area (i.e., all examined Austrian Federal Forests (ÖBf) forest enterprises). The width of bars along the x-axis represents the relative share of predisposition index classes among examined forest stands.
Simulation of Bark Beetle Development and Transpiration Deficits by PHENIPS-TDEF
The effective thermal sums DD-eff for bark beetle development clearly differed with topographical characteristics of the forest stands, particularly in elevation and parameters influencing potential global radiation, and the weather conditions of the observed season. As an example, thermal conditions at the mountainous (M) and lowland (LL) forest stands described in Table 3 ranged from DD-eff of 915 dd and 2690 dd in 2010 to DD-eff of 1413 dd and 2785 dd in 2015, respectively. On average, DD-eff were lowest across all forest stands of forest enterprises located in alpine areas such as OIT, UIT, PIG, and POG (Figure 5). Similarly, water balance showed high variability with regard to climate, geographical location and topography of sites, soil and stand structural characteristics. Yearly precipitation maxima of 1,706 and 1,862 mm were for instance recorded for TIV (2009) and PIG (2013), and minima of 455 and 499 mm for WW and WV (2015). Accordingly, mean yearly transpiration deficits simulated for management units ranged between a minimum of 1.06 mm (TIV, 2016) and a maximum of 96.5 mm (WW, 2015). Seasonal patterns of TDEF point to only minor drought events in earlier years (2008–2012) without pronounced transpiration deficits in any period of the season (Figure 2). From 2013 on, summer drought became more important than water deficits in spring and fall. This trend was particularly obvious for the forest enterprises WV and STM with 86–90% of deficits occurring from June to August in 2015.
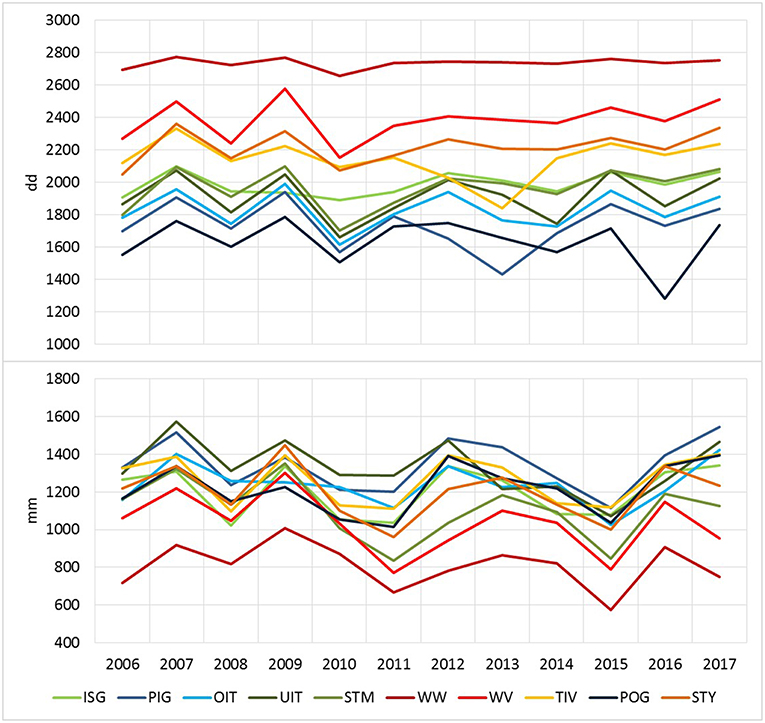
Figure 5. Climatic conditions at ÖBf forest enterprises with regard to effective thermal sums available for I. typographus development (upper graph) and yearly sums of precipitation (lower graph) averaged over forest stands.
Environmental Drivers of Bark Beetle Attack
Classification Trees
We built classification trees (CTs) for each ÖBf forest enterprise separately, taking into account observation periods of various length (Table S3). While not all of the CTs were significant (i.e., showing an AUC > 0.5), CTs built for PIG, POG, STM, TIV, WW, and WV (period 2014–2016, Figure 6) classified attacked and non-attacked stands correctly. Classification trees built for UIT, OIT and ISG predicted non-attack correctly, but not attack. In summary, the various CTs based on datasets of single forest enterprises explained an absence of infestation in 98.2–99.5%, and presence of attack in 8.5–32.7% of forest stands correctly (comp. Table S3). Linking the datasets of several ÖBf forest enterprises, presence of bark beetle attack was predicted correctly by 5.8% (absence by 99.5%) with regard to the year 2015 (Figure 7) despite highly variable stand and climate-related parameters and attack trends across the study areas.
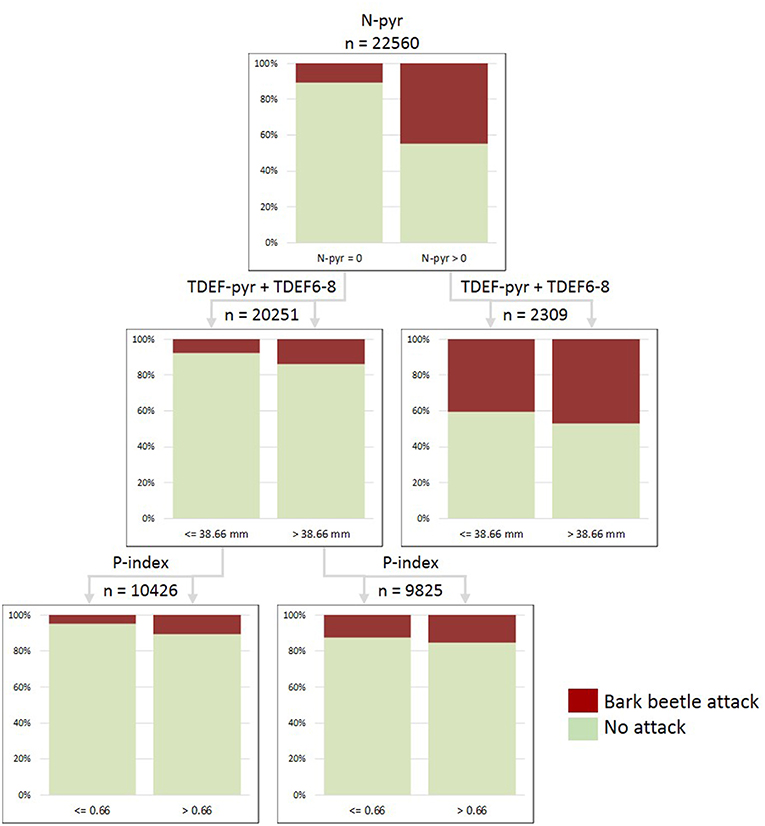
Figure 6. Classification tree “Waldviertel-Voralpen” (WV) built for the period 2014–2016 and including the variables: salvage loggings in the previous year (N-pyr), transpiration deficits accumulated in the previous year and June to August of the actual season (TDEF-pyr+TDEF6-8), and stand predisposition index (P-index). Total number of examined stands x observation years =22,560; proportions of correct predictions: 84.7% in total, 32.2% presence, 93.4% absence of attacks.
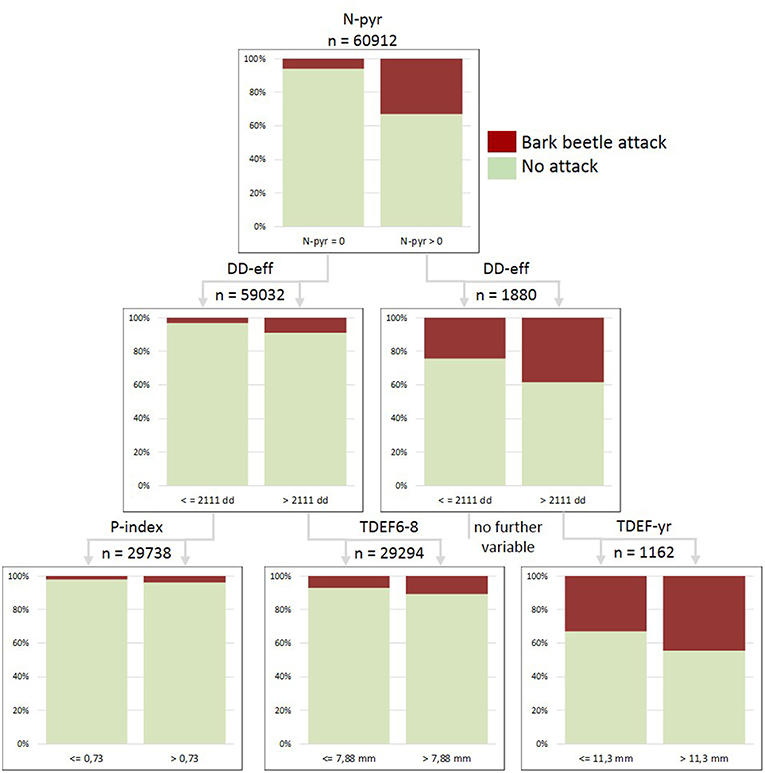
Figure 7. Classification tree for the merged dataset of forest enterprises built for the year 2015 and including the variables: salvage loggings in the previous year (N-pyr), effective thermal sums for bark beetle development (DD-eff), stand predisposition index (P-index), transpiration deficits accumulated from June to August of the actual season (TDEF6-8), and transpiration deficits accumulated from January to December of the actual season (TDEF-yr). Total number of examined stands x observation years =60,912; proportions of correct predictions: 93.1% in total, 5.8% presence of attacks, 99.5% absence of attacks.
The variable N-pyr (i.e., timber salvaged due to bark beetle attack in the previous year) was consistently ranked first in the hierarchical structure of all CTs (Figures 6, 7; Table S3). In the collective of forest stands subject to infestations, the proportion of continued bark beetle attacks in the following year was 26.6–42.7% higher than for novel attacks in previously unaffected forest stands across the observation period 2009–2016 (Table S3). Stand predisposition index was ranked second in the hierarchy of most CTs. For thresholds of P-index >0.7–0.8, proportions of both, new and continued attacks were up to 9.7% higher compared to the less predisposed stands. This relationship was particularly significant at the forest enterprise WW, where salvage loggings due to bark beetle infestation were continued in 44.2% of highly predisposed but only in 28.4% of less predisposed stands. The variables N-pyr and P-index alone explained 95.6/23.5% of absent/present bark beetle attack in Norway spruce rich stands of WW.
Transpiration deficits accumulated in the preceding year were identified as important predictors of bark beetle attack within CTs built for WV and STM since 2009 (Table S3). Compared to collectives of forest stands without transpiration deficits in the previous year, novel and continued attacks occurred in 1.7 and 9.1% more of the stands when TDEF-pyr thresholds of 5 mm were exceeded. Looking at the shorter observation period 2014–2016, the variable TDEF-pyr was also relevant in CTs of PIG, POG, and TIV, with 9.9–33.9% more continued attacks in drought-affected forest stands. Late season transpiration deficits (TDEF9-12) were relevant only for TIV. Acute summer transpiration deficits (TDEF6-8) turned out to be more significant in forest enterprises geographically located in lowland areas, such as WV. Here, proportions of infested forest stands ranged 8.2% <14.2% <35.0% within TDEF6-8 categories “below 20 mm” < “20–40 mm” < “above 40 mm” in the period 2009–2016. Consideration of the sum of TDEF-pyr and TDEF6-8 in CTs of WV and STM improved prediction accuracies, with proportions of correctly associated attacked stands of 32.7 and 16.2%, respectively (Table S3). Thresholds for this combined parameter indicating a probability of increased attack were identified at >10.9 mm (STM) and >38.7 mm (WV, Figure 6) within the observation period 2014–2016.
We further analyzed causal relationships for the increase in bark beetle-induced salvage loggings in 2015 for 60,912 forest stands in 10 ÖBf forest enterprises, and identified the following thresholds and hierarchical positions of parameters (Figure 7): (1) N-pyr (>0 m3 timber), (2) DD-eff (>2111 dd), (3) P-index (>0.73), (3) TDEF6-8 (>7.88 mm), (3) TDEF-yr (>11.34 mm). Novel infestation by I. typographus in 2015 ranged from a proportion of only 2.0% in the collective of forest stands characterized by comparably lower P-index and DD-eff to a proportion of 10.7% for stands subject to increased temperatures and acute summer drought (left path of Figure 7). Continued bark beetle attack was most probable (44.4%) for forest stands already subject to salvage harvests in the previous year, when effective thermal sums allowed for the development of at least two bark beetle generations and sister broods (DD-eff > 2,111 dd), and when transpiration deficits accumulated from January to December exceeded 11.3 mm.
Binary Logistic Regression
All parameters relevant in the classification trees except for TDEF9-12 were also significant in binary logistic regressions (Table 6). Using the merged dataset of ÖBf forest enterprises, the amount of salvage loggings due to bark beetle infestation was most influenced by the variables P-index, DD-eff, TDEF6-8, and N-pyr-factor within the period 2014–2016. The same parameter set also explained trends of salvaged timber since 2009 for WV. The binary logistic model for STM did confirm the important role of transpirations deficits accumulated in the previous year but not of actual summer drought as indicated in the CT. No serious residual problems were identified in the qq-plot or residuals vs. predicted plot. Likewise, Kolmogorov-Smirnov tests to explore overall scaled residual uniformity and the overdispersion test based on those residuals were not significant.
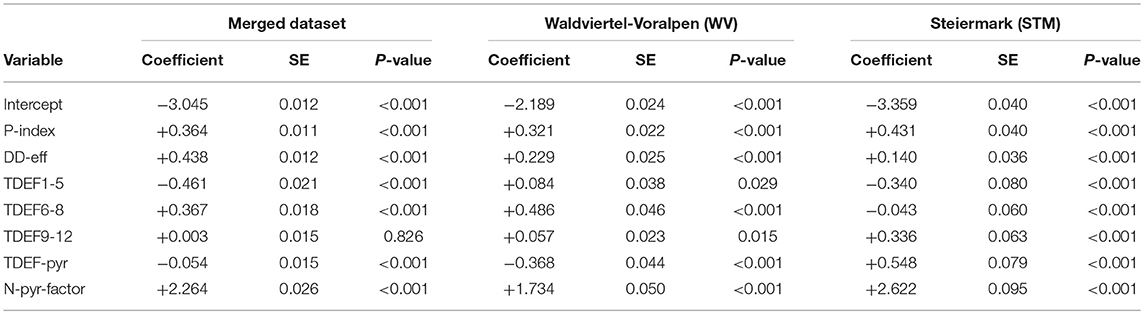
Table 6. Binary logistic regression models for predicting the absence/presence of bark beetle attack (N-yr-factor) for ÖBf forest enterprises: merged dataset (AUC = 0.720; R = 0.137), “Waldviertel-Voralpen” (WV) (AUC = 0.723; R = 0.157), and “Steiermark” (STM) (AUC = 0.720; R = 0.132) with regard to the observation period 2014–2016.
Visualization of causal relationships by use of 3D plots underline interactions between the most important environmental parameters in the models (Figure 8; Figures S4, S5). We found that increases in both temperature (DD-eff) and summer drought (TDEF6-8) or transpiration deficits accumulated over the previous year (TDEF-pyr) enhance the probability of bark beetle attack. Bark beetle infestation in the previous year (N-pyr) and high levels of P-index, either individually or in combination, further predispose forest stands to infestation by I. typographus.
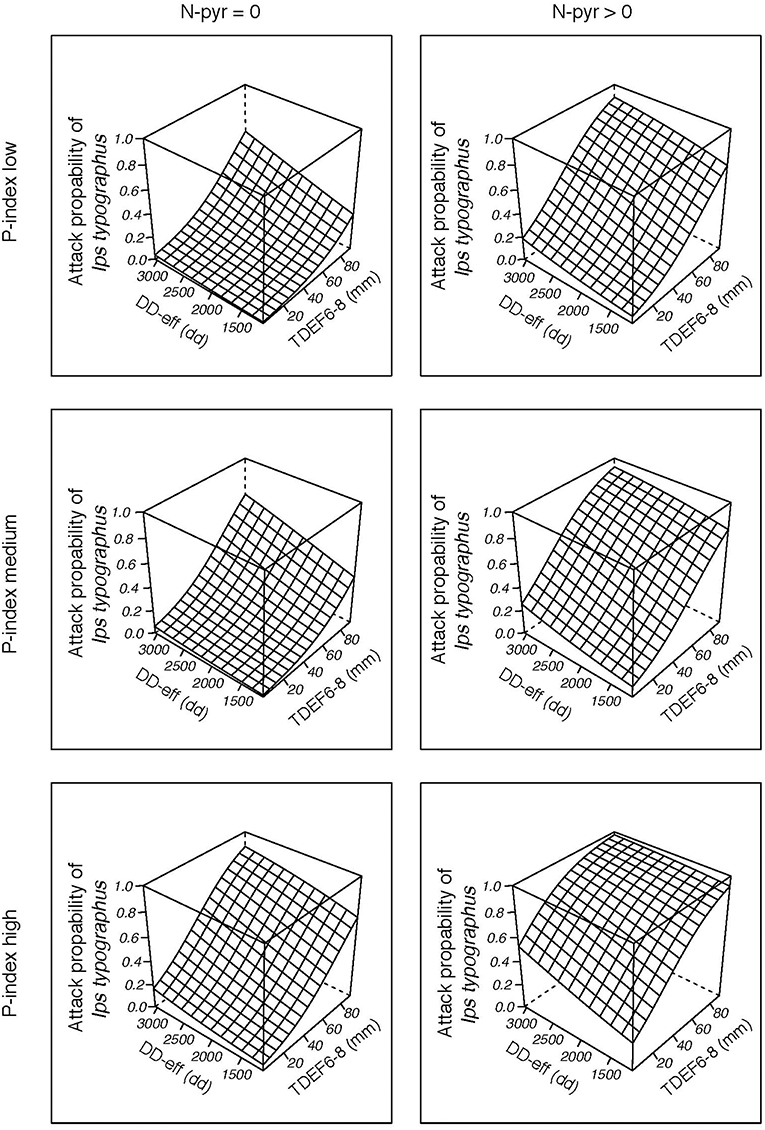
Figure 8. Probability of bark beetle attack for “Waldviertel-Voralpen” (WV) (2014–2016) as a function of effective thermal sum for I. typographus attack (DD-eff), transpiration deficits from June to August (TDEF6-8) accumulated in the current year, the occurrence of salvage loggings in the preceding year (N-pyr) and category of stand predisposition index (smallest/ medium/ highest P-index). The remaining, scaled variables of the binary logistic regression function were set to their means (=zero).
Discussion
Salvage logging due to bark beetle infestation since the year 2008 followed differential trends in the examined ÖBf enterprises; however, a distinct increase in intensity since 2015 was observed across the whole study area. The absence of major storm damage events in the observation period allowed us to focus on the climatic, non-windthrow drivers of bark beetle disturbance, in particular drought episodes. As underlined by Bentz et al. (2019), cross-regional drought stress associated with warm temperature conditions can initiate mass-attack of host stands by I. typographus and D. ponderosae, both bark beetle species showing high reproductive capacity. This assumption is supported by this study of spatially explicit simulations of bark beetle phenology and stand transpiration deficits using the novel model combination PHENIPS-TDEF (Matthews et al., 2018). The relevance of drought stress for infestation was mediated by population dynamics as indicated by salvage logging trends and stand predisposition index. Although stand transpiration deficits were most strongly related to I. typographus infestation in lowland areas outside natural Norway spruce habitats such as the forest enterprise WV, TDEF variables were still found to be significant in classification trees and binary logistic regression built to explain recent outbreaks for the whole study region. The diverging influence of acute drought (in terms of recent transpiration deficits) and chronic drought conditions (naturally given by shallow forest sites of low soil moisture) on infestation probability, might explain why drought was previously found to be less relevant in the study area (Pasztor et al., 2014).
Incidence of Bark Beetle Attack Depending on Site Water Supply and P-Index
Chronically dry forest stands, described in the ÖBf forest inventory as growing on shallow, xeric, and low moisture soil conditions, were significantly less prone to bark beetle attack across all forest enterprises (Figures 3A–C). This at first sight unexpected trend was most pronounced for forest enterprises with a majority of low elevation Norway spruce stands such as TIV and WV, and is possibly due to an adaptation of spruce trees to soils of limited water storage. Long-term but moderate drought stress has been hypothesized to increase tree resistance to phloem feeding insects as more energy resources are invested in the synthesis of defensive, secondary metabolites (Ayres and Lombardero, 2000; Jactel et al., 2012). Vice versa, enhanced tree growth due to restoration measures aiming at a reduction of tree competition and change in tree species composition of secondary pure Norway spruce stands has been shown to be associated with reduced defense capability against bark beetles (Baier et al., 2002). Similarly, stands growing on fertile sites were found to be most susceptible to severe infestation as found in a Finnish study (Blomqvist et al., 2018). While a high number of primary beetle attacks is required to overcome resistance of vital Norway spruce (Christiansen et al., 1987), the nutrient rich phloem of these trees eventually promotes brood development (Lindenthal and Führer, 1993). I. typographus in fact prefers vigorously growing trees that suffer from sudden drought stress (Führer et al., 1997). Acute water deficits of Norway spruce stands where soil conditions are usually moist is reported to trigger infestation by I. typographus, especially in lowland areas (Holuša et al., 2018). ÖBf forest stands situated in precipitation rich, natural distribution areas of Norway spruce were most of the time well water supplied and therefore less exposed to severe drought periods leading to acute tree stress.
Distribution of predisposition index (P-index) classes proved to be biased toward high values indicating closed Norway spruce stands of increased age in High Mountain/alpine forest enterprises in particular. Despite comparably low amounts of timber salvaged due to bark beetle attack in higher elevation forest stands over the entire observation period, the more recent increase in I. typographus infestation was still significantly concentrated in forest stands of elevated stand-related predisposition (Figure 4). These results underline the general importance of the considered stand properties as indicators of stand susceptibility for Norway spruce forests in and outside their natural habitat (Netherer and Nopp-Mayr, 2005; Hilszczanski et al., 2006). Correspondingly, standing volume of Norway spruce, a variable aggregating several stand parameters similar to P-index, strongly affected the occurrence of infestation spots in forest districts all over Switzerland (Stadelmann et al., 2013). Spruce abundance has furthermore been demonstrated to be positively correlated with frequency of bark beetle attack in the Bavarian Forest National Park (Lausch et al., 2011).
Simulations by PHENIPS-TDEF
Here, we present for the first time results based on the combined simulation of bark beetle phenology and stand transpiration deficits by PHENIPS-TDEF performed for a multitude of forest stands on a regional scale which were diverse in forest composition, structure and site conditions. At the original study site where the TDEF model was demonstrated, the model reproduced measured soil water potential, volumetric soil water content and tree transpiration (Matthews et al., 2018). Although the water balance module would certainly benefit from further calibration and validation against hydrological datasets of other mature Norway spruce stands, in our present work we assumed applicability of the process-based model for a general comparison of various site conditions. As PHENIPS simulations have repeatedly been demonstrated to correspond to bark beetle flight and development observed in a number of studies (Baier et al., 2007; Berec et al., 2013; Netherer et al., 2015; Fleischer et al., 2016), the calculated effective thermal sums DD-eff can be regarded as reliable indicators of maximum potential bark beetle generation development. Geographic location of each forest enterprise influencing the altitudinal range of forest stands within had clear impact on thermal conditions for bark beetle development. At low elevation, sun-exposed sites, a third filial generation potentially developed in all years of the period 2014–2017. Such thermally favored lowland and sub-mountainous forest stands clearly predominated in the forest enterprises WW, WV, TIV, and STY as shown by seasonal DD-eff above 2,000 dd accumulated on average across all management units (Figure 5). Quite contrary, the harsh climatic conditions at some high elevation forest stands of PIG, POG and OIT did at times not even allow for the termination of one filial generation. It is noteworthy that the calculated effective temperature sums for filial and sister brood establishment remained more or less stable within regions, pointing to the fact that there was no distinct warming trend over the period 2006–2017 that would allow for the establishment of further generations. Although positive temperature fluctuations in the observation period did not always come along with precipitation deficits, high DD-eff were principally associated with low precipitation sums at forest enterprises of lower altitudes and vice versa in the alpine regions. While 2015 was one of the driest years, mean yearly amounts of rainfall strongly varied between drought prone areas of WW and WV (572, 787 mm) and precipitation-rich PIG or TIV (1,114, 1,119 mm).
The water balance module TDEF used for the simulation of stand transpiration deficits relies on coupled atmosphere and soil hydrological processes; yet, was intentionally kept simple to facilitate practical application in risk assessment of I. typographus infestations in Norway spruce forests (Matthews et al., 2018). Neither snow dynamics nor water fluxes according to differentiated soil horizons, which are standard procedures in complex soil-plant-atmosphere systems such as the COUP model (Jansson and Karlberg, 2011) and Brook 90 (Federer et al., 2003), were considered. The straightforward concept of the model, however, allowed us to derive the necessary coarse above-ground and below-ground description of forest stands of interest from forest inventory datasets originally not established for scientific analyses. The constraints of the present approach lie in the uncertainty of how accurately TDEF input variables describe actual stand parameters. Although heterogeneity of site and stand characteristics is likely to increase with forest stand size, such diversity was not expressed in the ÖBf forest inventories, which only summarize the predominant tree species composition, stand age and structure, and soil parameters of a management unit. A further concern, similar for all studies based on forest inventory data, is the continuous change in the spatial arrangement of stand polygons and stand structural features due to planned and unplanned forest management activities, which is recognized through revision of the dataset every 10 years only (Pasztor et al., 2014; Mezei et al., 2017). Furthermore, not all parameters important for simulating transpiration deficits by TDEF, for instance LAI, were directly available from the forest inventory database but had to be deduced based on functional relationships or by interpretation of qualitative information. In the TDEF model the amount of precipitation intercepted, evaporated and falling through, respectively, is influenced by the canopy's dryness and the maximum amount of water the canopy can store, which depends on the proportion of the forest floor covered by the canopy (Matthews et al., 2018). Up to 60% of precipitation is potentially intercepted by a spruce stand, whereby interceptive and transpiration surface and thus, potential dryness of soil increases with leaf area and stand density (Alavi et al., 2001). By considering a range of LAI values between 4 and 8 depending on forest stand age, we took into account the non-constant vegetation/evapotranspiration processes over time important in a hydrologic model (Yu et al., 2014), also including a continuous decrease in leaf area with advanced stand age due to needle loss and forest gaps (Pokorný and Stojnic, 2012).
The simulation of forest stand water supply and potential drought stress requires essential knowledge on principal relationships between soil water potential and volumetric soil-water content influenced by soil physical properties (Rehschuh et al., 2017; Novak and Hlavacikova, 2019). Availability of accurate soil hydraulic properties on a larger scale is generally limited across Europe, in particular with regard to forest soils, but is commonly compensated in models by the use of pedo-transfer functions (Wösten et al., 1999). Pedo-transfer functions are empirical, regression-type relationships developed from local and/or regional datasets to estimate coefficients of hydrologic equations, most often those of Brooks and Corey (1964) and Van Genuchten (1984). We derived the necessary variables “saturated water content,” “residual water content,” “air entry pressure head,” and “unimodal pore size distribution” from the functions of Rawls and Brakensiek (1989) with the limitation that soil texture, rooting depth and volumetric stone content had to be interpreted from qualitative ÖBf site descriptions. While we strongly recommend parametrization of TDEF or other hydrologic models with site-specific input parameters in the case of small-scale studies, we regard this approximation adequate for this comparison of water supply/deficits of the >80,000 forest stands distributed across highly diverse site conditions. A set of point-related soil mapping data available for the forest enterprises UIT and OIT (Waldtypisierung Tirol, 2019) was used for a first validation of texture classes (Table S6). Simulated transpiration deficits strongly differed between forest enterprises, observation years and spring, summer or fall season. Despite substantial amounts of yearly precipitation, distinct spring and in particular summer drought episodes repeatedly stressed Norway spruce stands even in cooler/wetter High Mountain or alpine regions. From 2013 onwards, summer deficits (TDEF6-8) overall increased, amounting to over 90% of yearly transpiration deficits in several ÖBf forest enterprises.
Environmental Drivers of Bark Beetle Attack
We decided for the statistical methods applied to explain bark beetle infestation in management units of ÖBf forest enterprises for several reasons. Non-parametric classification trees (CT) have proved as practical tools for testing habitat suitability of protected species (Zohmann et al., 2014) and I. typographus infestation risk (Zolubas et al., 2009) due to their robustness concerning variable distributions, scale levels, and multi-collinearities of predicting variables (De'ath and Fabricius, 2000). They are suited to select a significant subset of predictors from a larger set of variables for use in building a formal parametric model. The method allows for the creation of rules, i.e., thresholds of the chosen predictors, to predict future events such as bark beetle attack of forest stands, thereby identifying homogeneous groups with higher or lower risk. All variables relevant in the here established CTs also proved to be significant in the binary logistic models, which corroborated the importance of selected predictors for the assessment of infestation probabilities. Both models, however, have their limitations. Similar to modeling habitat suitability for desired species, not all environmental and intraspecific parameters playing a role in the predisposition of forests to bark beetle attack can be covered (Schweiger et al., 2011), e.g., because data are not available or the relationships are just unknown. In our study, we lacked direct information on I. typographus population dynamics, for instance indicated by pheromone trap catches, number or density of infested trees or offspring. Reliable representation of beetle abundance by traps would require long time series and a dense net of records (Okland and Berryman, 2004; Schroeder, 2013), not feasible to be established area-wide; yet, the amount of salvage logged timber considered here can at least provide a rough estimate of attack densities. We furthermore did not take into account stand structural features within management units that acutely increase the risk of bark beetle attacks, such as sun-exposed stand edges and gaps newly created by harvesting activities or abiotic damage events, due to the limitations of the forest inventory dataset discussed previously. The probability of wind damage is strongly affected by topography, elevation, slope, and aspect (Stadelmann et al., 2014), not considered here, as well as by stand characteristics. In the High Tatra Mountains, about 75% of variation in wind and bark beetle induced mortality in the course of a severe outbreak could be explained by site characteristics also including global radiation and pH of soil on the one hand, and stand age, height and diameter (Mezei et al., 2014) on the other hand. Analyzing an alternative Austrian forest disturbance database, Thom et al. (2013) related 18.7% of variation in bark beetle damage records (2002–2010) to wind disturbance and infestation observed in the previous 2 years. Pasztor et al. (2014) investigated drivers of bark beetle infestation in the ÖBf forest enterprises TIV, STY, WV, and STM for the period 1992–2010, which provided the opportunity to compare composition and relevance of parameter sets. While the earlier study considered a more comprehensive list of site and stand related attributes but similarly took into account the variables examined here (including a simple soil moisture index for evaluating stand water supply), predictions were comparably accurate. Values of R2 in equal measure ranged 0.13–0.16 and 0.05–0.17 in our and earlier models, respectively. Such “weak” linear relationships might be inter alia due to relevant predictors still unknown or the high frequency of zero valued observations indicating an absence of salvage logging activities in the majority of forest stands independent from predisposing environmental parameters. Nevertheless, sensitivity, i.e., power to identify positive predictions, reached a maximum of 32.7% cases correctly identified by CTs established for WV in the present study as compared to 29% in the logistic regression model of Pasztor et al. (2014). Analogous to their results, we found susceptibility of stands to bark beetle attack increase with the share of Norway spruce, stand age and density (high P-index), and follow-up damages becoming much more likely with precedent infestation.
P-Index and N-pyr were the main predictors of bark beetle attacks in each of the established CTs (Figures 6, 7; Table S3) and binary logistic regression models (Table 4). Salvage logging performed in the previous year was consistently ranked highest priority, resulting in maxima of >40% more attacks compared to hitherto unaffected forest stands in years of increasing bark beetle disturbance. In this respect, outbreak patterns do not seem to differentiate so much from protected Norway spruce rich forests such as the High Tatras national park, where progressive tree mortality has mainly been driven by distance to previous-year infestation, particularly during peak epidemic phases (Potterf et al., 2019). Favorable environmental conditions further trigger mass propagation of beetles, promoting fast and repeated attack of new trees within one season, and thereby posing a challenge to forest managers in timely detection and removal of infested timber. The remaining positive relationship of N-pyr and presence/probability of infestation over the entire observation period of almost a decade points to the high availability of susceptible trees in the study area that still enables population growth far below the threshold of resource depletion and rapid population decline (Okland and Bjørnstad, 2006; Marini et al., 2017). I. typographus outbreaks have resulted in landscape level mortality of Norway spruce (Kautz et al., 2011), especially when triggered by storm damage, but are driven to a high degree by tree health status in case of other inciting factors such as increased temperature and drought (De Groot et al., 2019).
Stand transpiration deficits clearly turned out to be decisive predictors of recent years' bark beetle infestation in Austrian Norway spruce stands with particular importance for forest enterprises geographically located in warmer and drier areas of the country. The sum of previous year and actual summer deficits was classified second highest priority in CT WV, the region most severely affected by the ongoing calamity, independent from salvage loggings, and in CT STM with relevance for stands where previous year attacks have continued. Regarding the clear increase in salvaged timber since 2015 for the majority of ÖBf forest enterprises, this trend was explained best (apart from N-pyr) by thermal conditions allowing for at least two filial and two sister brood generations as well as transpiration deficits accumulated over the entire year. TDEF-yr ≥11 mm and TDEF6-8 ≥ 8 mm separating less from more strongly affected management areas are not regarded as universally valid thresholds but suggest that infestation risks might rise at even low yearly or summer transpiration deficits. Generalized linear models substantiated the relative importance of predictors by indicating a rising relevance of effective thermal sums and summer drought (TDEF6-8) for the probability of I. typographus infestation with increasing P-index and N-pyr across all forest enterprises, but particularly in regions sensitive to drought (Figure 8). At WV, proportions of salvaged stands considerably increased with summer transpiration deficits ≥40 mm, which reflects the potential relevance of drought with respect to future disturbance regimes, but also the importance of geographical region and weather conditions.
Conclusions
We found that a combination of ample host availability, favorable temperature conditions for bark beetle development, and acute disposition of trees to attack caused by drought stress can intensify population growth and most likely lead to bark beetle mass outbreaks in the absence of severe abiotic disturbance. This is alarming in view of the projected increased exposure of temperate climate zones to drought events and associated sensitivity of conifer forests to biotic disturbance (Lindner et al., 2010). Despite high vulnerability to water deficits in terms of cavitation risks, northern populations of Pinus spp., and P. abies seem to lack adequate strategies of xylem and stomatal control in order to protect against hydraulic constraints (Isaac-Renton et al., 2018; Peters et al., 2018). For the first time directly relating tree transpiration deficits to attack by I. typographus, Matthews et al. (2018) found clear hints for compromised tree defense. Building upon this finding, we took a step forward toward identifying specific thresholds of drought stress, being fully aware of study limitations with regard to methods, geographic area and observation period. We therefore consider further, broad experimental research on relationships between I. typographus infestation risk and the transpiration deficit or other drought stress proxies indispensable in order to complement and refine systems for a reliable monitoring and assessment of I. typographus infestation risks in Norway spruce stands. Such monitoring can strongly support forest managers in the prioritization and scheduling of pest control measures with particular regard to detection of susceptible stands and timely removal of infested trees. In areas likely to be subject to increased incidence of acute drought, adaptive and prophylactic measures such as promotion of alternative tree species is strongly recommended.
Data Availability
The datasets generated for this study will be made available on request by the corresponding author.
Author Contributions
SN conceived the study and had the leading role in generating datasets from forest inventory and infestation data, statistical analyses, preparation, review, and revision of the manuscript. BP provided expertise in generalized linear modeling, prepared figures in R, and thoroughly reviewed the manuscript. JP processed the geodatabase and climate data, modeled bark beetle phenology and transpiration deficits, and contributed to manuscript preparation. BM provided support in model implementation and reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Austrian Federal Forests, ÖBf AG financed this study. We thank Martin Schebeck for comments on parts of an earlier version of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2019.00039/full#supplementary-material
Footnotes
References
Alavi, G., Jansson, P.-E., Hällgren, J.-E., and Bergholm, J. (2001). Interception of a dense spruce forest, performance of a simplified canopy water balance model. Nordic Hydrol. 32, 265–284. doi: 10.2166/nh.2001.0016
Annila, E. (1969). Influence of temperature upon the development and voltinism of Ips typographus L. (Coleoptera, Scolytidae). Ann. Zool. Fenn. 6, 161–208.
Ayres, M. P., and Lombardero, M. J. (2000). Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 262, 263–286. doi: 10.1016/S0048-9697(00)00528-3
Baier, P., Führer, E., Kirisits, T., and Rosner, S. (2002). Defence reactions of Norway spruce against bark beetles and the associated fungus Ceratocystis polonica in secondary pure and mixed species stands. Forest Ecol. Manag. 159, 73–86. doi: 10.1016/S0378-1127(01)00711-3
Baier, P., Pennerstorfer, J., and Schopf, A. (2007). PHENIPS—A comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. Forest Ecol. Manag. 249, 171–186. doi: 10.1016/j.foreco.2007.05.020
Bentz, B. J., Jönsson, A. M., Schroeder, M., Weed, A., Wilcke, R. A. I., and Larsson, K. (2019). Ips typographus and dendroctonus ponderosae models project thermal suitability for intra- and inter-continental establishment in a changing climate. Front. Forests Glob. Change 2:1. doi: 10.3389/ffgc.2019.00001
Berec, L., Doležal, P., and Hais, M. (2013). Population dynamics of Ips typographus in the Bohemian Forest (Czech Republic): validation of the phenology model PHENIPS and impacts of climate change. Forest Ecol. Manag. 292, 1–9. doi: 10.1016/j.foreco.2012.12.018
BFW (2014). Einführung in die Bodenkartierung. Austrian Research Centre for Forests. Available online at: https://bfw.ac.at/300/pdf/Einfuehrung_Bodenkartierung.pdf (accessed January 17, 2019).
Blomqvist, M., Kosunen, M., Starr, M., Kantola, T., Holopainen, M., and Lyytikäinen-Saarenmaa, P. (2018). Modelling the predisposition of Norway spruce to Ips typographus L. infestation by means of environmental factors in southern Finland. Eur. J. Forest Res. 137, 675–691. doi: 10.1007/s10342-018-1133-0
Bréda, N., Huc, R., Granier, A., and Dreyer, E. (2006). Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann. Forest Sci. 63, 625–644. doi: 10.1051/forest:2006042
Breuer, L., Eckhardt, K., and Frede, H.-G. (2003). Plant parameter values for models in temperate climates. Ecol. Model. 169, 237–293. doi: 10.1016/S0304-3800(03)00274-6
Brooks, R. H., and Corey, A. T. (1964). “Hydraulic properties of porous media,” in Colorado State University Hydrology Papers. Colorado State University, Fort Collins, CO, United States, 37.
Christiansen, E., Waring, R. H., and Berryman, A. A. (1987). Resistance of conifers to bark beetle attack: searching for general relationships. Forest Ecol. Manag. 22, 89–106. doi: 10.1016/0378-1127(87)90098-3
Coops, N. C., Wulder, M. A., and Waring, R. H. (2012). Modeling lodgepole and jack pine vulnerability to mountain pine beetle expansion into the western Canadian boreal forest. Forest Ecol. Manag. 274, 161–171. doi: 10.1016/j.foreco.2012.02.011
De Groot, M., Diaci, J., and Ogris, N. (2019). Forest management history is an important factor in bark beetle outbreaks: Lessons for the future. Forest Ecol. Manag. 433, 467–474. doi: 10.1016/j.foreco.2018.11.025
De'ath, G., and Fabricius, K. E. (2000). Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81, 3178–3192. doi: 10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2
Dodson, R., and Marks, D. (1997). Daily air temperature interpolated at high spatial resolution over a large mountainous region. Climate Res. 8, 1–20. doi: 10.3354/cr008001
Dymond, C. C., Wulder, M. A., Shore, T. L., Nelson, T., Boots, B., and Riel, B. G. (2006). Evaluation of risk assessment of mountain pine beetle infestations. Western J. Appl. Forestry 21, 5–13. doi: 10.1093/wjaf/21.1.5
Faccoli, M. (2009). Effect of weather on Ips typographus (Coleoptera Curculionidae) phenology, voltinism, and associated spruce mortality in the southeastern Alps. Environ. Entomol. 38, 307–317. doi: 10.1603/022.038.0202
Federer, C. A., Vörösmarty, C., and Fekete, B. (2003). Sensitivity of annual evaporation to soil and root properties in two models of contrasting complexity. J. Hydrometeorol. 4, 1276–1290. doi: 10.1175/1525-7541(2003)004<1276:SOAETS>2.0.CO;2
Fettig, C. J., Klepzig, K. D., Billings, R. F., Munson, A. S., Nebeker, T. E., Negrón, J. F., et al. (2007). The effectiveness of vegetation management practices for prevention and control of bark beetle infestations in coniferous forests of the western and southern United States. Forest Ecol. Manag. 238, 24–53. doi: 10.1016/j.foreco.2006.10.011
Fleischer, P., Fleischer, P., Ferenčík, J., Hlaváč, P., and Kozánek, M. (2016). Elevated bark temperature in unremoved stumps after disturbances facilitates multi-voltinism in Ips typographus population in a mountainous forest. Forestry J. 62, 15–22. doi: 10.1515/forj-2016-0002
Führer, E., Lindenthal, J., and Baier, P. (1997). Baummortalität bei Fichte: zusammenhänge zwischen prämortaler Vitalitätsdynamik und dem Befall durch rindenbrütende Insekten. Mitteilungen der Dtsch.Ges.Allg.Angew.Ent. 11, 645–648.
Gower, S. T., and Norman, J. M. (1991). Estimation of Leaf Area Index in conifer and broad-leaf plantations. Ecology 72, 1896–1900. doi: 10.2307/1940988
Hanewinkel, M., Hummel, S., and Albrecht, A. (2010). Assessing natural hazards in forestry for risk management: a review. Eur. J. Forest Res. 130, 329–351. doi: 10.1007/s10342-010-0392-1
Hartig, F. (2018). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.2.0 ed. University of Regensburg.
Hilszczanski, J., Janiszewski, W., Negron, J., and Munson, A. S. (2006). Stand characteristics and Ips typographus (L.) (Col., Curculionidae, Scolytinae) infestation during outbreak in northeastern Poland. Folia Forestalia Polonica Series A For. 48, 53–64.
Hlasny, T., Zajickova, L., Turcani, M., Holusa, M., and Sitkova, Z. (2011). Geographical variability of spruce bark beetle development under climate change in the Czech Republic. J. For. Sci. 57, 242–249. doi: 10.17221/104/2010-JFS
Holuša, J., Lubojacký, J., Curn, V., Tonka, T., Lukášová, K., and Horák, J. (2018). Combined effects of drought stress and Armillaria infection on tree mortality in Norway spruce plantations. For. Ecol. Manag. 427, 434–445. doi: 10.1016/j.foreco.2018.01.031
Hosmer, D. W., and Lemeshow, S. (2000). Applied Logistic Regression. New York, NY: John Wiley and Sons. doi: 10.1002/0471722146
Iio, A., and Ito, A. (eds) (2014). A Global Database of Field-Observed Leaf Area Index in Woody Plant Species, 1932–2011. Oak Ridge, TN: Oak Ridge National Laboratory Distributed Active Archive Center.
Isaac-Renton, M., Montwé, D., Hamann, A., Spiecker, H., Cherubini, P., and Treydte, K. (2018). Northern forest tree populations are physiologically maladapted to drought. Nat. Commun. 9:5254. doi: 10.1038/s41467-018-07701-0
Jactel, H., Petit, J., Desprez-Loustau, M.-L., Delzon, S., Piou, D., Battisti, A., et al. (2012). Drought effects on damage by forest insects and pathogens: a meta-analysis. Glob. Change Biol. 18, 267–276. doi: 10.1111/j.1365-2486.2011.02512.x
Jansson, P.-E., and Karlberg, L. (2011). COUP Manual: Coupled Heat and Mass Transfer Model for Soil-Plant-Atmosphere Systems. Stockholm.
Jönsson, A. M., Appelberg, G., Harding, S., and Bärring, L. (2009). Spatio-temporal impact of climate change on the activity and voltinism of the spruce bark beetle,Ips typographus. Glob. Change Biol. 15, 486–499. doi: 10.1111/j.1365-2486.2008.01742.x
Jönsson, A. M., Harding, S., Krokene, P., Lange, H., Lindelöw, Å. K., Økland, B., et al. (2011). Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapause. Climatic Change 109, 695–718. doi: 10.1007/s10584-011-0038-4
Jönsson, A. M., and Lagergren, F. (2018). Effects of climate and soil conditions on the productivity and defence capacity of Picea abies in Sweden—An ecosystem model assessment. Ecol. Model. 384, 154–167. doi: 10.1016/j.ecolmodel.2018.06.023
Kautz, M., Dworschak, K., Gruppe, A., and Schopf, R. (2011). Quantifying spatio-temporal dispersion of bark beetle infestations in epidemic and non-epidemic conditions. For. Ecol. Manag. 262, 598–608. doi: 10.1016/j.foreco.2011.04.023
Köstner, B., Falge, E., and Tenhunen, J. D. (2002). Age-related effects on leaf area/sapwood area relationships, canopy transpiration and carbon gain of Norway spruce stands (Picea abies) in the Fichtelgebirge, Germany. Tree Physiol. 22, 567–574. doi: 10.1093/treephys/22.8.567
Lange, H., Okland, B., and Krokene, P. (2006). Thresholds in the life cycle of the spruce bark beetle under climate change. Inter J. Complex Syst. 1648, 1–10.
Laubhann, D., Eckmüllner, O., and Sterba, H. (2010). Applicability of non-destructive substitutes for leaf area in different stands of Norway spruce (Picea abies L. Karst.) focusing on traditional forest crown measures. For. Ecol. Manag. 260, 1498–1506. doi: 10.1016/j.foreco.2010.07.048
Lausch, A., Fahse, L., and Heurich, M. (2011). Factors affecting the spatio-temporal dispersion of Ips typographus (L.) in Bavarian Forest National Park: a long-term quantitative landscape-level analysis. For. Ecol. Manag. 261, 233–245. doi: 10.1016/j.foreco.2010.10.012
Lindenthal, J., and Führer, E. (1993). Rindenbrüter-Befallsspektren Und Prämortale Vitalitätsdynamik Natürlich Abgestorbener Fichten. FIW-Forschungsberichte. Vienna: University of Natural Resources and Life Sciences.
Lindner, M., Maroschek, M., Netherer, S., Kremer, A., Barbati, A., Garcia-Gonzalo, J., et al. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 259, 698–709. doi: 10.1016/j.foreco.2009.09.023
Lobinger, G. (1994). Air temperature as a limiting factor for flight activity of two species of pine bark beetles, Ips typographus L. and Pityogenes chalcographus L. (Col., Scolytidae). Anzeiger f€ur Sch€adlingskunde Pflanzenschutz Umweltschutz 67, 14–17. doi: 10.1007/BF01906563
Logan, J. A., White, P., Bentz, B. J., and Powell, J. A. (1998). Model analysis of spatial patterns in mountain pine beetle outbreaks. Theor. Popul. Biol. 53, 236–255. doi: 10.1006/tpbi.1997.1350
Marini, L., Lindelöw, Å., Jönsson, A. M., Wulff, S., and Schroeder, L. M. (2013). Population dynamics of the spruce bark beetle: a long-term study. Oikos 122, 1768–1776. doi: 10.1111/j.1600-0706.2013.00431.x
Marini, L., Økland, B., Jönsson, A. M., Bentz, B., Carroll, A., Forster, B., et al. (2017). Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 40, 1426–1435. doi: 10.1111/ecog.02769
Matthews, B., Netherer, S., Katzensteiner, K., Pennerstorfer, J., Blackwell, E., Henschke, P., et al. (2018). Transpiration deficits increase host susceptibility to bark beetle attack: experimental observations and practical outcomes for Ips typographus hazard assessment. Agri. For. Meteorol. 263, 69–89. doi: 10.1016/j.agrformet.2018.08.004
Mezei, P., Grodzki, W., BlaŽenec, M., and Jakuš, R. (2014). Factors influencing the wind–bark beetles' disturbance system in the course of an Ips typographus outbreak in the Tatra Mountains. For. Ecol. Manag. 312, 67–77. doi: 10.1016/j.foreco.2013.10.020
Mezei, P., Jakuš, R., Pennerstorfer, J., Havašová, M., Škvarenina, J., Ferenčík, J., et al. (2017). Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—an infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agri. For. Meteorol. 242, 85–95. doi: 10.1016/j.agrformet.2017.04.004
Monteith, J. L., and Unsworth, M. H. (2008). Principles of Environmental Physics. Oxford: Academic Press.
Nagelkerke, N. J. D. (1991). A note on a general definition of the coefficient of determination. Biometrika 78, 691–692. doi: 10.1093/biomet/78.3.691
Negrón, J. F., Allen, K., Cook, B., and Withrow, J. R. (2008). Susceptibility of ponderosa pine, Pinus ponderosa (Dougl. ex Laws.), to mountain pine beetle, Dendroctonus ponderosae Hopkins, attack in uneven-aged stands in the Black Hills of South Dakota and Wyoming USA. For. Ecol. Manag. 254, 327–334. doi: 10.1016/j.foreco.2007.08.018
Netherer, S., Ehn, M., Blackwell, E., and Kirisits, T. (2016). Defence reactions of mature Norway spruce (Picea abies) before and after inoculation of the blue-stain fungus Endoconidiophora polonica in a drought stress experiment. For. J. 62, 169–177. doi: 10.1515/forj-2016-0014
Netherer, S., Matthews, B., Katzensteiner, K., Blackwell, E., Henschke, P., Hietz, P., et al. (2015). Do water-limiting conditions predispose Norway spruce to bark beetle attack? N. Phytol. 205, 1128–1141. doi: 10.1111/nph.13166
Netherer, S., and Nopp-Mayr, U. (2005). Predisposition assessment systems (PAS) as supportive tools in forest management—rating of site and stand-related hazards of bark beetle infestation in the High Tatra Mountains as an example for system application and verification. For. Ecol. Manag. 207, 99–107. doi: 10.1016/j.foreco.2004.10.020
Netherer, S., Pennerstorfer, J., and Matthews, B. (2019). Drought stress predisposes Norway spruce stands to attack by the Eurasian spruce bark beetle. Forstschutz Aktuell 65, 1–9.
Novak, V., and Hlavacikova, H. (2019). “Basic physical characteristics of soils,” in Theory and Applications of Transport in Porous Media, ed S. M. Hassanizadeh (Switzerland: Springer Nature), 15–28. doi: 10.1007/978-3-030-01806-1_2
Okland, B., and Berryman, A. A. (2004). Resource dynamic plays a key role in regional fluctuations of the spruce bark beetles Ips typographus. Agri. For. Entomol. 6, 141–146. doi: 10.1111/j.1461-9555.2004.00214.x
Okland, B., and Bjørnstad, O. N. (2006). A resource-depletion model of forest insect outbreaks. Ecology 87, 283–290. doi: 10.1890/05-0135
Panassiti, B., Breuer, M., Marquardt, S., and Biedermann, R. (2013). Influence of environment and climate on occurrence of the cixiid planthopper Hyalesthes obsoletus, the vector of the grapevine disease 'bois noir'. Bull. Entomol. Res. 103, 621–633. doi: 10.1017/S0007485313000163
Pasztor, F., Matulla, C., Rammer, W., and Lexer, M. J. (2014). Drivers of the bark beetle disturbance regime in Alpine forests in Austria. For. Ecol. Manag. 318, 349–358. doi: 10.1016/j.foreco.2014.01.044
Peters, R. L., Speich, M., Pappas, C., Kahmen, A., Von Arx, G., Graf Pannatier, E., et al. (2018). Contrasting stomatal sensitivity to temperature and soil drought in mature alpine conifers. Plant Cell Environ. 42, 1674–1689. doi: 10.1111/pce.13500
Pokorný, R., and Stojnic, S. (2012). Leaf area index of Norway spruce stands in relation to age and defoliation. Beskydy 5, 173–180. doi: 10.11118/beskyd201205020173
Potterf, M., Nikolov, C., Kočická, E., Ferenčík, J., Mezei, P., and Jakuš, R. (2019). Landscape-level spread of beetle infestations from windthrown- and beetle-killed trees in the non-intervention zone of the Tatra National Park, Slovakia (Central Europe). For. Ecol. Manag. 432, 489–500. doi: 10.1016/j.foreco.2018.09.050
R Core Team (2014). R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rawls, W. J., and Brakensiek, D. L. (1989). “Estimation of soil water retention and hydraulic properties,” in Unsaturated Flow in Hydrologic Modelling: Theory and Practice, ed H. J. Morel-Seytoux (Dordrecht: Springer), 275–300. doi: 10.1007/978-94-009-2352-2_10
Rehschuh, R., Mette, T., Menzel, A., and Buras, A. (2017). Soil properties affect the drought susceptibility of Norway spruce. Dendrochronologia 45, 81–89. doi: 10.1016/j.dendro.2017.07.003
Robertson, C., Wulder, M. A., Nelson, T. A., and White, J. C. (2008). Risk rating for mountain pine beetle infestation of lodgepole pine forests over large areas with ordinal regression modelling. For. Ecol. Manag. 256, 900–912. doi: 10.1016/j.foreco.2008.05.054
Safranyik, L., Carroll, A. L., Régnière, J., Langor, D. W., Riel, W. G., Shore, T. L., et al. (2012). Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can. Entomol. 142, 415–442. doi: 10.4039/n08-CPA01
Schelhaas, M. J., Nabuurs, G. J., and Schuck, A. (2003). Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Change Biol. 9, 1620–1633. doi: 10.1046/j.1365-2486.2003.00684.x
Schroeder, L. M. (2013). Monitoring ofIps typographusandPityogenes chalcographus: influence of trapping site and surrounding landscape on catches. Agr. For. Entomol. 15, 113–119. doi: 10.1111/afe.12002
Schurman, J. S., Trotsiuk, V., Bace, R., Cada, V., Fraver, S., Janda, P., et al. (2018). Large-scale disturbance legacies and the climate sensitivity of primary Picea abies forests. Glob. Chang Biol. 24, 2169–2181. doi: 10.1111/gcb.14041
Schweiger, A.-K., Nopp-Mayr, U., and Zohmann, M. (2011). Small-scale habitat use of black grouse (Tetrao tetrix L.) and rock ptarmigan (Lagopus muta helvetica Thienemann) in the Austrian Alps. Eur. J. Wildlife Res. 58, 35–45. doi: 10.1007/s10344-011-0537-7
Seidl, R., Baier, P., Rammer, W., Schopf, A., and Lexer, M. J. (2007). Modelling tree mortality by bark beetle infestation in Norway spruce forests. Ecol. Model. 206, 383–399. doi: 10.1016/j.ecolmodel.2007.04.002
Seidl, R., Fernandes, P. M., Fonseca, T. F., Gillet, F., Jönsson, A. M., Merganičová, K., et al. (2011). Modelling natural disturbances in forest ecosystems: a review. Ecol. Model. 222, 903–924. doi: 10.1016/j.ecolmodel.2010.09.040
Seidl, R., Müller, J., Hothorn, T., Bässler, C., Heurich, M., and Kautz, M. (2015). Small beetle, large-scale drivers: how regional and landscape factors affect outbreaks of the European spruce bark beetle. J. Appl. Ecol. 53, 530–540. doi: 10.1111/1365-2664.12540
Senf, C., and Seidl, R. (2018). Natural disturbances are spatially diverse but temporally synchronized across temperate forest landscapes in Europe. Glob. Change Biol. 24, 1201–1211. doi: 10.1111/gcb.13897
Soil Survey Division Staff (1993). Soil Survey Manual. Soil Conservation Service, U.S. Department of Agriculture Handbook.
Stadelmann, G., Bugmann, H., Wermelinger, B., and Bigler, C. (2014). Spatial interactions between storm damage and subsequent infestations by the European spruce bark beetle. For. Ecol. Manag. 318, 167–174. doi: 10.1016/j.foreco.2014.01.022
Stadelmann, G., Bugmann, H., Wermelinger, B., Meier, F., and Bigler, C. (2013). A predictive framework to assess spatio-temporal variability of infestations by the European spruce bark beetle. Ecography 36, 1208–1217. doi: 10.1111/j.1600-0587.2013.00177.x
Temperli, C., Bugmann, H., and Elkin, C. (2013). Cross-scale interactions among bark beetles, climate change, and wind disturbances: a landscape modeling approach. Ecol. Monogr. 83, 383–402. doi: 10.1890/12-1503.1
Thom, D., Seidl, R., Steyrer, G., Krehan, H., and Formayer, H. (2013). Slow and fast drivers of the natural disturbance regime in Central European forest ecosystems. For. Ecol. Manag. 307, 293–302. doi: 10.1016/j.foreco.2013.07.017
Van Genuchten, M. T. (1984). “Management aspect for crop production,” in Soil Salinity Under Irrigation: Processes and Management, eds I. Shainberg and J. Shalhevet (Berlin, Heidelberg: Springer), 258–338. doi: 10.1007/978-3-642-69836-1_8
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics With S. New York, NY: Springer. doi: 10.1007/978-0-387-21706-2
Wermelinger, B. (2004). Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For. Ecol. Manag. 202, 67–82. doi: 10.1016/j.foreco.2004.07.018
Wermelinger, B., and Seifert, M. (1998). Analysis of the temperature dependent development of the spruce bark beetle lps typographus (L.) (Col., Scolytidae). J. Appl. Entomol. 122, 185–191. doi: 10.1111/j.1439-0418.1998.tb01482.x
Wermelinger, B., and Seifert, M. (1999). Analysis of the temperature dependent development of the spruce bark beetle lps typographus (L.) (Col., Scolytidae). Ecol. Entomol. 24, 103–110. doi: 10.1046/j.1365-2311.1999.00175.x
Wösten, J. H. M., Lilly, A., Nemes, A., and Le Bas, C. (1999). Development and use of a database of hydraulic properties of European soils. Geoderma 90, 169–185. doi: 10.1016/S0016-7061(98)00132-3
Yu, X., Lamačová, A., Duffy, C., Krám, P., Hruška, J., White, T., et al. (2014). Modelling long-term water yield effects of forest management in a Norway spruce forest. Hydrol. Sci. J. 60, 174–191. doi: 10.1080/02626667.2014.897406
Zohmann, M., Immitzer, M., Wöss, M., Gossow, H., and Nopp-Mayr, U. (2014). Modelling habitat use of Tetrao urogallus L. in Austria for conservation issues. J. Nat. Conserv. 22, 223–234. doi: 10.1016/j.jnc.2014.01.002
Keywords: Ips typographus, bark beetles, Picea abies, risk assessment, drought stress, transpiration deficit, modeling
Citation: Netherer S, Panassiti B, Pennerstorfer J and Matthews B (2019) Acute Drought Is an Important Driver of Bark Beetle Infestation in Austrian Norway Spruce Stands. Front. For. Glob. Change 2:39. doi: 10.3389/ffgc.2019.00039
Received: 09 April 2019; Accepted: 02 July 2019;
Published: 17 July 2019.
Edited by:
Barry Alan Gardiner, European Forest Institute, FinlandReviewed by:
Tomáš Hlásny, Czech University of Life Sciences Prague, CzechiaKevin J. Dodds, United States Forest Service, United States
Copyright © 2019 Netherer, Panassiti, Pennerstorfer and Matthews. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sigrid Netherer, sigrid.netherer@boku.ac.at
 Sigrid Netherer
Sigrid Netherer Bernd Panassiti
Bernd Panassiti Josef Pennerstorfer
Josef Pennerstorfer Bradley Matthews
Bradley Matthews