Less-Studied Technology-Critical Elements (Nb, Ta, Ga, In, Ge, Te) in the Marine Environment: Review on Their Concentrations in Water and Organisms
- 1Marine Biogeochemistry Group, Instituto de Investigacións Mariñas-CSIC, Vigo, Spain
- 2Marine Pollution and Biological Effects, Instituto Español de Oceanografía, San Pedro del Pinatar, Spain
The development in recent years of new technological and energy-related applications has increased the use and demand of a specific group of trace elements (Technology-Critical Elements, TCEs). Among the TCEs, there are a number of elements (Nb, Ta, Ga, In, Ge, Te) for which their biogeochemical cycles and their ecotoxicology and uptake by biota has been scarcely studied; they are known as Less-Studied TCEs (LSTCEs). Here we present a review on the concentrations of LSTCEs in marine waters and biota. We show that whereas oceanic profiles have been reported for all LSTCEs, and their geochemical behavior is well-constrained for some of them (e.g., Ga, In, Ge), only very few studies are available on the concentrations and behavior of these elements in estuarine and coastal waters which makes impossible the assessment of their status in environmentally impacted coastal areas. In marine biota, despite of the fact that concentrations have been reported in several organisms, information on the factors controlling the LSTCEs uptake or their potential to be biomagnified through the food web is mostly missing. It is therefore encouraged further research in order to have a better assessment on the impact of the uses of these metals on the concentrations of LSTCEs in such sensitive coastal zones, including their concentrations, bioavailability, thresholds for non-lethal endpoints and their capability of biomagnification.
Introduction
The Earth’s crust is composed of a great variety of igneous, metamorphic and sedimentary rocks, mainly dominated by a few elements (e.g., Si, Al, Fe, Ca, Na, K, Mg) and their oxides, comprising approximately 99.7% of the crust (Rudnick and Gao, 2003). The majority of the naturally occurring chemical elements (minor and trace elements) account only for the remaining 0.3%. The deleterious effects of some of the trace elements to living organisms have been well documented and have led to their concentrations in the aquatic environment to be regulated by protocols and directives (i.e., EU Water Framework Directive 2000/60/CE and 2008/56/CE) that determine their appropriate environmental concentrations.
A significant number of trace elements are, however, excluded in these studies. This is due to: (i) their low ambient concentrations, generally below the detection limits of the analytical procedures employed, and (ii) the absence of any significant industrial role in the past, thus having no apparent environmental implications (Cobelo-García et al., 2015). This situation is currently changing, since several of these non-regulated trace elements are now key components in the development of new technologies, including electronic displays, semiconductors, energy–related technologies or telecommunications technology. They are defined as Technology-Critical Elements (TCEs1), and include tellurium (Te), germanium (Ge), gallium (Ga), indium (In), niobium (Nb), tantalum (Ta), the platinum group elements (PGEs: Pt, Pd, Rh, Os, Ir, Ru) and the rare earth elements (REEs: La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Yb, Lu). The extent of the environmental impact of the increasing anthropogenic use of these technology–critical elements needs therefore to be further assessed. Among the TCEs, the group of the less-studied TCEs (LSTCEs) – comprising Te, Ge, Ga, In, Nb and Ta – was defined by Filella and Rodríguez-Murillo (2017) for those TCEs for which, from an environmental perspective, have been less studied than the REEs or PGEs. A major reason for this scarcity in environmental studies of LSTCEs relies in the fact that their analytical determination is still challenging (e.g., Filella and Rodushkin, 2018).
Here we present a review on the available information existing to date on the concentrations of the LSTCEs in the marine environment, specifically in the water column and marine organisms. Based on the data collected, we provide an evaluation of the current gaps in our knowledge of these elements in marine systems and indicate directions for future research.
Occurrence in Nature and Anthropogenic Applications
Niobium and Tantalum
Niobium and Ta are metals belonging to the Group 5 of the Periodic Table of Elements, and together with the adjacent metals Zr, Hf, Mo and W are known as ‘high field strength elements’ (HFSE; Rudnick and Gao, 2003). Both elements form compounds with a variety of oxidation states, but the + 5 is the only environmentally relevant (Filella, 2017). Niobium has only one natural stable isotope (93Nb), but several artificial unstable isotopes that are found in radioactive wastes such as 95Nb (35 days half-life) and 94Nb (20,300 years half-life) (Astrom et al., 2008). Tantalum has two natural isotopes, the stable 181Ta (99.988%) and the metastable 180Ta (0.012%) (Filella, 2017). According to Rudnick and Gao (2003), the average concentrations of Nb and Ta in the upper continental crust are 12 and 0.9 μg g–1, respectively. Niobium and Ta are considered as chemical twins due to their similar chemical properties (i.e., ionic charge and radius) generating a coherent behavior during magmatic differentiation; as a results, the Nb/Ta ratios are expected to be constant and of chondritic value (∼17) in mantle and mantle-derived rocks (Hui et al., 2011; Niu, 2012). However, several studies have observed a large Nb/Ta fractionation in rocks from the world ocean floor; the causes for such unexpected fractionation are still under debate (Niu, 2012). The strongest fractionation observed for the Nb/Ta occurs in the aquatic environment, particularly in seawater; in general, the Nb/Ta ratios increase in the following order: continental crust < river water < coastal water < open ocean (Firdaus et al., 2008).
These elements are usually found together in nature, with coltan (short for columbite-tantalites) as the most commonly known ore of Nb–Ta. The world mine production for these metals has significantly increased in the past two decades, currently accounting for roughly 60,000 (Nb) and 1,200 (Ta) tons per year (Filella and Rodríguez-Murillo, 2017). Both elements are used in a variety of high-tech applications, including high-grade structural steel and superalloys (e.g., aerospace industry), and capacitators in several electronic devices (e.g., medical appliances, portable electronics, flat-screen TVs, etc.; Cobelo-García et al., 2015; Filella, 2017).
Gallium and Indium
Gallium and In are close neighbors in the periodic table and both have the + 3 as their only oxidation state in the environment. They have two natural isotopes each, 69Ga (60.11%) and 71Ga (39.89%), and 115In (95.72%) and 113In (4.28%). Their typical concentrations in the upper continental crust are 17.5 (Ga) and 0.056 (In) μg/g (Rudnick and Gao, 2003).
The use of Ga in semiconductors has made it a high-tech metal in the past decades. The two main application fields are integrated circuits and optoelectronic devices. The most commonly used Ga compound is gallium-arsenide (GaAs), followed by gallium-nitride (GaN); other compounds (e.g., gallium-antimonide – GaSb – and gallium-phosphide – GaP) are used in much smaller amounts. By mid-2010’s, the global Ga consumption was around 285 t, representing a 70% increase compared to late 2000’s, and an increasing demand is foreseeing during the coming years (Rongguo et al., 2016). The In production by mid-2010’s was estimated to be around 1,500 t, being the liquid crystal display (LCD) industry in the manufacturing of flat-panel, touch-screen, and plasma displays for televisions, computers, and handheld electronic devices the main In application (>50% of the total consumption) in the form of ITO (Indium Tin Oxide). By 2020, the In demand is expected to increase over 2000 t (Lokanc et al., 2015).
Germanium
Germanium (Ge) has 5 naturally occurring isotopes: 70Ge (20.8%), 72Ge (27.5%), 73Ge (7.7%), 74Ge (36.3%), and 76Ge (7.6%). This element shows a similar geochemical behavior to that of Si, as it has similar electronic configuration and ionic radii (Lewis et al., 1988), but unlike Si it has no known essential function for organisms (Höll et al., 2007). It has an abundance in the upper continental crust of ∼1.4 μg/g (Rudnick and Gao, 2003), and the common oxidation state in natural systems is +4, forming at natural pHs in solution the hydroxide Ge(OH)4 as its main species.
Germanium is used in fiber-optic systems, infrared optics and in electronics and solar electric applications. It is mainly obtained as a by-product during Zn mining and production; by mid-1990’s, the Ge world production was ∼60 tons per year, and has increased since then to reach around 160 tons per year by 2015 (Filella and Rodríguez-Murillo, 2017).
Tellurium
Tellurium has eight natural isotopes (120Te, 122Te, 123Te, 124Te, 125Te, 126Te, 128Te, 130Te), the most abundant being 130Te (34.1%) and 128Te (31.7%). Tellurium (Te) belongs to the chalcogen group, which also includes O, S and Se. Unlike the other chalcogen elements, Te has not any known biological role (Nolan et al., 1991; Belzile and Chen, 2015). Te can exist in nature in various redox states: telluride (−2), elemental Te (0), tellurite (+ 4) and tellurate (+ 6). Under typical environmental conditions (e.g., oxygen, pH, etc.), the most abundant redox form of Te are Te4+ and Te6+ (Filella et al., 2019). They exist mainly as oxyanions and/or hydroxides, although their main chemical species are still a matter of controversy (Filella et al., 2019). The classical publications on elemental crustal abundance do not provide values for Te; values given for the Te terrestrial abundance display a range from 2 to 27 ng/g (Filella et al., 2019).
The main use of Te is for the production of cadmium–telluride (CdTe), which is used in semiconductor materials due to its optical and electrical properties especially in solar cells (Ramos-Ruiz et al., 2016). The solar cells industry is estimated to account for 40% of the global Te demand, followed by the thermoelectric production (30%; USGS, 2018). The estimation of the global Te production in 2017 was 410 tons, which is a 3–4 fold increase with respect to two decades ago – this increase being driven by its demand in the photovoltaic and thermoelectric applications (Filella et al., 2019).
Concentrations and Behavior in the Marine Environment
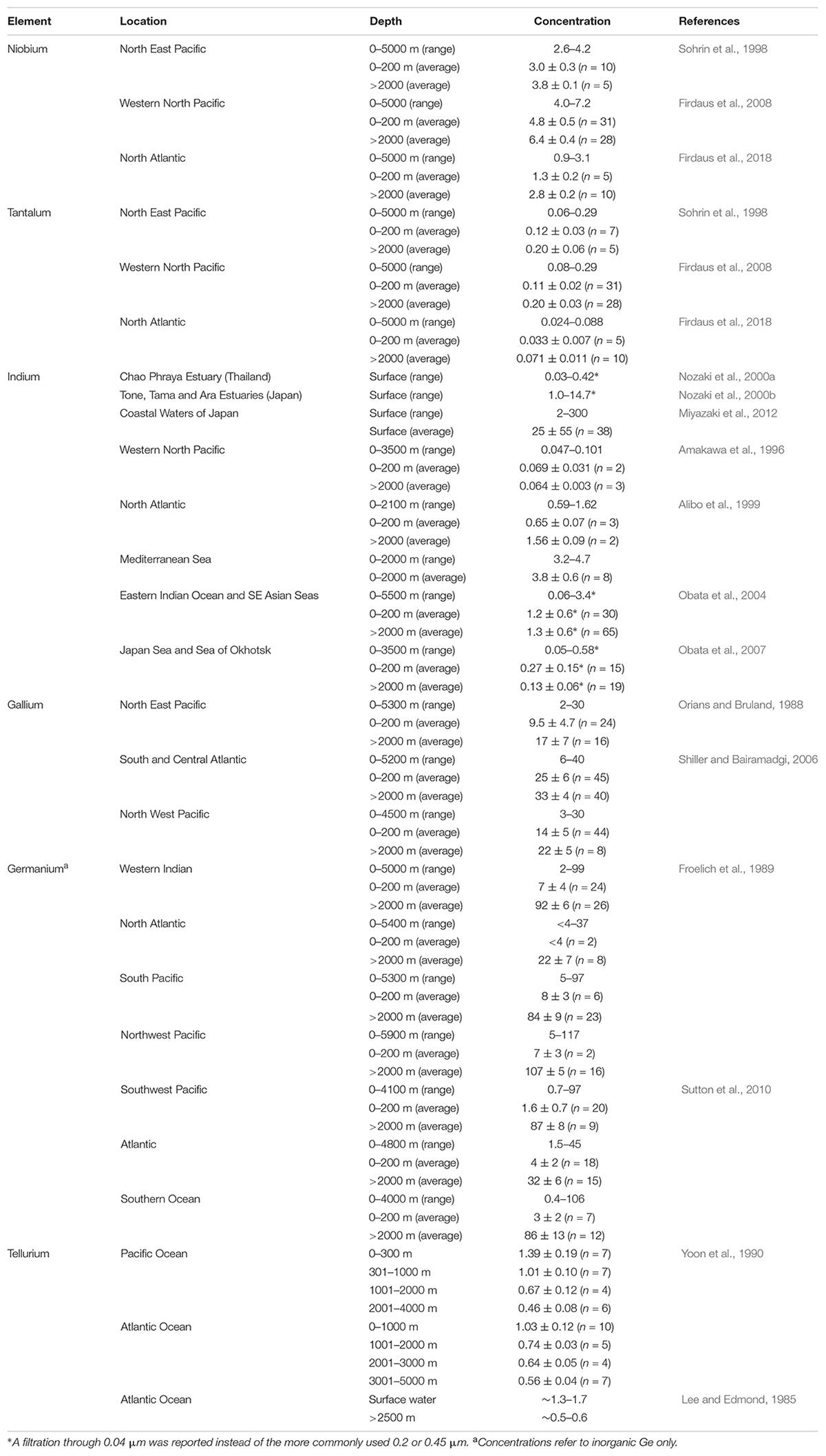
Concentrations of dissolved Nb in the world’s oceans range from ∼1 to 7 pmol/kg (Table 1), and from ∼ 0.02 to 0.3 pmol/kg for Ta. Both elements are depleted in surface waters and show a deep water enrichment (Table 1; Firdaus et al., 2011, 2018), indicating a removal in the photic zone by carrier phases and bottom water enrichment due to sediment resuspension (Firdaus et al., 2008). Contrary to open ocean waters, higher concentrations of Nb and Ta were observed in surface waters of coastal stations (Andaman Sea and Gulf of Thailand; Firdaus et al., 2018) and decreasing toward deep waters. This was attributed to the dissolution effect of atmospheric particles and riverine inputs (Firdaus et al., 2018).
The main dissolved inorganic forms of Ga and In in seawater are the hydroxylated species Me(OH)30 and Me(OH)4–, as derived from thermodynamic calculations (Byrne et al., 1988). The hydrolysis products of trivalent metals display a significant particle-reactivity in the oceans, thus leading to short residence times (Alibo et al., 1999; Obata et al., 2007). Accordingly, Alibo et al. (1999) found the acid-soluble particulate fraction of In the Mediterranean to average 47 ± 11% (n = 6), which is typical of other particle-reactive metals as Ce or Th. Thus, their concentrations are generally maintained at low concentrations due to removal from seawater by scavenging onto particles (Alibo et al., 1999). Both elements are geochemically similar to aluminum, although less particle-reactive (Al/Ga and Al/In in seawater are two orders of magnitude smaller than that of shale; Amakawa et al., 1996), and have the potential to provide information on dust input to the surface ocean that complements that obtained using Al as a tracer (Shiller and Bairamadgi, 2006).
Elevated In concentrations (up to 300 pM) compared to open ocean waters were reported for Japanese coastal waters (Table 1), especially those close to industrialized ports (Miyazaki et al., 2012). Also, evidence for In contamination was found in several estuaries in Japan (Tama, Ara, and Tone estuaries; Nozaki et al., 2000b); here dissolved In followed a smooth decrease with salinity and was significantly correlated with Gd – which presented a positive anomaly due to its use of Gd-DTPA as a medical agent in magnetic resonance imaging in hospitals. Nozaki et al. (2000b) suggested that In could be anthropogenically derived in a soluble form similar to that of Gd and pointed to the use of In(DTPA)2– as a diagnostic medical agent as a possible explanation. For Ga, concentrations of 11,000–19,000 pM were obtained for a coastal stream in Taiwan receiving wastewater treatment plant discharges impacted by a science park housing semiconductor, electronics and optoelectronic manufacturing (Hsu et al., 2011), and is therefore a potential Ga source to the neighboring coastal waters.
Criteria continuous concentrations (CCC) for Ga and In – defined as the highest concentration of a toxicant to which aquatic organisms can be exposed indefinitely without causing unacceptable effects –were predicted by use of quantitative ion characteristic-activity relationships-species sensitivity distributions (QUICAR-SSD; Mu et al., 2014; Qie et al., 2017); this method, used to predict the biological activity of metal ions, is based on the assumption that similar electronic configurations should have similar functions and therefore several physico-chemical descriptors are applied for such calculations. For In, the calculated CCC of 5 nM (Qie et al., 2017) to 120 nM (Mu et al., 2014) are at least one order of magnitude higher than the highest dissolved In observed in the marine environment (0.3 nM in Japanese coastal waters, see above). In the case of Ga, a predicted CCC of 200 nM was reported (Mu et al., 2014). However, based on chronic toxicity data for several marine species, van Dam et al. (2018) proposed a marine water quality guideline (WQG) for this element of 11.5 μM, i.e., 2 orders of magnitude higher than the value proposed by Mu et al. (2014). In any case, the highest reported Ga concentrations in impacted estuarine-coastal areas (<20 nM; see above) are below both guideline values.
The Ga/In ratios in seawater are in the range of those in shale, indicating a similar chemical reactivity in the oceans, although some fractionation during takes place during scavenging resulting in different vertical profiles (Amakawa et al., 1996). Both Ga and In show a large interoceanic variation, with concentrations in the Atlantic considerably higher than in the Pacific (Table 1; Alibo et al., 1999; Shiller and Bairamadgi, 2006). Solubilization of In from aeolian dust – rather than riverine and/or coastal waters – has been suggested as the main source of In to the oceans, and would explain the elevated concentrations observed in the Mediterranean Sea (Table 1) due to the higher dust flux in this basin. Aeolian dust deposition also represents the main Ga delivery to the surface ocean at a global scale, although inputs from river plumes may be important in certain coastal areas (e.g., Columbia river plyme; McAlister and Orians, 2012).
In natural waters dissolved Ge is present as inorganic Ge (Gei) in the form of Ge(OH)4, monomethyl germanium (MMGe) and dimethylgermanium (DMGe) (Lewis et al., 1988). The geochemical behavior of Gei closely resembles that of Si, showing a strong correlation (Froelich et al., 1989; Santosa et al., 1997; Ellwood and Maher, 2003), as they are cycled in seawater by diatoms. However, fractionation between Si and Ge has been observed during uptake by and regeneration from phytoplankton, which may explain the slight positive Ge intercepts seen for the global Ge versus Si relationships (Ellwood and Maher, 2003; Sutton et al., 2010). In estuaries, Gei displays a non-conservative behavior, showing both net inputs and removal following the seasonal Si cycle (Andreae et al., 1983; Froelich et al., 1985a, b). Unlike Gei, MMGe and DMGe display and apparent stable and inert behavior, producing conservative profiles in estuaries and the ocean (Lewis et al., 1988) in contrast with methylated species for other metals such as As (e.g., monomethylarsonic acid and dimethylarsinic acid) which show maximum concentrations in surface waters (Santosa et al., 1997). Experiments conducted by Lewis et al. (1989) suggested that methylgermanium species are produced on the continents during methanogenensis and derived to the oceans where they remain unreactive; however, Gei enrichment and methylgermanium deplation was observed in marine anoxic waters indicating that anaerobic processes are capable of demethylating marine organogermanium (Lewis et al., 1989).
Gei concentrations typically range from 0.5 to 120 pM, with lower concentrations in the surface layers reflecting diatom uptake. For MMGe and DMGe, a global average concentration of 330 ± 15 pM (MMGe) and 120 ± 20 pM (DMGe) has been proposed, and account for more than 70% of total dissolved Ge in seawater (Lewis et al., 1988). In estuaries, conservative behavior of MMGe and DMGE – reflecting their unreactive characteristics – was observed, with low concentrations in the freshwater end-member compared to seawater. For Gei, its distribution in estuaries is non-conservative (Froelich et al., 1985a, b) and also showing a similar behavior to Si as observed for the ocean.
A criteria continuous concentrations (CCC; see above) around 1 μM was predicted for Ge in the marine environment (Mu et al., 2014). Concentrations reported for Gei in estuaries and coastal areas (Andreae et al., 1983; Froelich et al., 1985a, b) are in the range of those found for the ocean, and are thus several orders of magnitude lower that these quality limits. Elevated Ge concentrations in coastal watersheds were only reported in areas impacted by effluents by the leather industry, which contains high amounts of Ge (Zhang and Zhang, 2007).
The only two studies reporting an oceanic Te profile provided a total dissolved Te ranging from ∼0.4 to ∼2 pM (Table 1), showing a scavenged-type behavior profile in the water column. The two studies cited in Table 1 were published in 1985 and 1990 (no other oceanic profiles has been reported since) and, as noted in the extensive review of Filella et al. (2019), their reported concentrations are 2–3 orders of magnitude lower than those provided in more recent studies for seawater. Accordingly, a total Te concentration of 305 pM was given for a coastal water in the English Channel (Biver et al., 2015), with 70% as Te(VI). Concentrations of 85–263 pM were obtained for coastal seawater close to cities in China, with Te(VI) representing 70–87% of total Te (Huang and Hu, 2008). For the Changjiang Estuary and nearby coastal waters, Wu et al. (2014) reported concentrations of Te(IV) ranging from 3 to 60 pM, and from 5 to 330 for Te(VI), observing a predominance of Te(IV) in surface waters and Te(VI) in bottom waters. A similar Te(VI) predominance over Te(IV) was found throughout the water column of the Atlantic and Pacific oceans (Yoon et al., 1990). Te(IV) predominance over Te(VI) was observed at the redox boundary of the Saanich Inlet (Canada) indicating that the reduced form may prevail under oxygen-depleted conditions; however, these inorganic Te forms were not the dominant species in this basin, and accounted only less than 30% of total Te, which suggests the formation of organotellurium species in these waters (Yoon et al., 1990).
Concentrations and Uptake by Marine Organisms
There are few data on Nb and Ta concentrations in marine organisms, although there is some evidence of a high accumulation coefficient for these elements by zooplankton in aquatic systems (Chebotina et al., 2011). Sánchez-Rodríguez et al. (2001) reported Ta concentrations ranging from 5 to 350 ng/g in different seaweed species collected in Loreto Bay (Gulf of California, Mexico). Using a dissolved Ta concentration in surface coastal waters of ∼0.2 pmol/kg (0.036 ng/kg), results in an enrichment factor (EF) of 105–107, indicating an elevated accumulation of this element by seaweed. Concentrations of Ta in invertebrates and fishes from coastal zones of Chile representing different climatic zones were reported by Espejo et al. (2018). In macroinvertebrates they found concentrations ranging from 0.17 to 7.8 ng/g, with a median value of 0.51 ng/g (n = 24), whereas for fishes ranged from 0.61 to 14.0 ng/g and a median of 2.4 ng/g (n = 22). Importantly, they observed a correlation between the Ta concentrations and the trophic levels for each of the coastal areas sampled indicating a Ta biomagnification through the aquatic food webs. In mussels (Mytilus Galloprovincialis) cultivated in rafts from the Galician rias (NW Iberian Peninsula), concentrations up to 211 ng/g were obtained, although average values were normally below the detection limit (<8 ng/g; Costas-Rodríguez et al., 2010). In this same study, average Nb concentrations ranged from <6 to 29 ng/g, with values as high as 235 ng/g observed. In the CRM DORM-2 (dog-fish muscle), Engström et al. (2004) provided a concentration of 2.8 ± 0.3 ng/g.
For In, we only found one published study reporting concentrations in marine organisms, namely the marine mussel tissue CRM NIST2976 (24 ± 5 ng/g; Krishna and Arunachalam, 2004) – probably reflecting the analytical difficulties in its determination and the fact that this metal is commonly used as an internal standard during the analytical determination by ICPMS. The situation is somewhat different for Ga, for which there are several studies reporting concentrations in biota. For example, Costas-Rodríguez et al. (2010) obtained concentrations ranging from 20 to 580 ng/g in mussels (Mytilus Galloprovincialis), with average values from 60 (Pontevedra Ria) to 240 ng/g (Vigo Ria). In two species of clams collected in the coastal Ganzirri Lake (Messina, Italy), Di Bella et al. (2013) reported concentrations of 610 ± 370 ng/g (range 380–1500 ng/g) for Venerupis aurea laeta and 370 ± 300 ng/g (range 140–880 ng/g) for Cerastoderma edule glaucum, with no significant differences in the concentrations between both species. In 32 fish species from the French market, Guérin et al. (2011) obtained an average Ga concentration of 2 ± 1 ng/g, and an average of 7 ± 12 ng/g in other seafood and products (n = 20). It is interesting noting that the value obtained by Guérin et al. (2011) for mussels (3 ng/g) is 1–2 orders of magnitude than those obtained from the Galician rias (Costas-Rodríguez et al., 2010); suggesting possible geographical or species-specific reasons explaining such differences in concentrations, although analytical bias cannot be excluded.
In fish from Malaysian coastal waters, Agusa et al. (2005) obtained Ga concentrations ranging from 9 to 469 ng/g in liver, and from <1 to 104 ng/g in muscle; here, the authors observed a strong positive correlation between concentrations in liver and muscle. Gallium concentrations in liver samples of dolphins collected in the Brazilian coast reported by Kunito et al. (2004) were 5 ± 2 ng/g (n = 20; Sotalia guianensis) and 3 ± 4 ng/g (n = 23; Pontoporia blainvillei), with no significant differences between both species and between immature and mature specimens. Campbell et al. (2005) analyzed a range of trace elements in a pelagic Arctic marine food web (North water Polynya, Baffin Bay, Canada), including primary organisms (e.g., ice algae), invertebrates, fish (cod), seabirds and mammals (ringed seal); for Ga they observed no relationship in its concentrations with the trophic position indicating that it was not biomagnified or biodiluted through the food web. They found, however, significant correlations of Ga between liver and muscle in ringed seals and seabirds – in accordance with the results given by Agusa et al. (2005) for fish –, indicating that this element is proportionally distributed throughout the body tissues.
Germanium concentrations in fish and seafood from French market ranged from 1 to 5 ng/g (Guérin et al., 2011), with an average value of 2 ± 2 ng/g (n = 52). One order of magnitude higher concentration (68 ± 5 ng/g) was reported for the marine mussel tissue CRM NIST2976 (Krishna and Arunachalam, 2004). For Te, Guérin et al. (2011) reported concentrations from 1 to 12 ng/g, with a mean value of 2–3 ng/g (n = 52) in fish. These values are in the range of those observed for mussels from the Galician rias, with concentration ranging from <1.4 to 5.9 ng/g (Costas-Rodríguez et al., 2010). Similar concentrations were reported in an historical Te record (1984–2017) in wild oysters from the Gironde Estuary (France); here Gil-Díaz et al. (2019) obtained an average concentration of 2.08 ng/g, with values ranging from 1.33 to 2.89 ng/g with no clear temporal trend unlike other anthropogenically released metals (e.g., Cd, Ag, Pt). These values are within those obtained in wild oysters for the Arcachon Bay (France; 1.18 ± 0.52 ng/g, n = 20) or the Bilbao Estuary (Spain; 3.48 ± 1.39 ng/g, n = 20) (Gil-Díaz et al., 2019).
Data on Te for two CRMs have been reported; accordingly, Engström et al. (2004) provided a concentration of 1.8 ± 0.1 ng/g for DORM-2 (dog-fish muscle) and Filella et al. (2019) a value of 5.5 ± 0.9 ng/g for BCR-414 (plankton). Tellurium was also analyzed in different tissues of the squid Todarodes pacificus in the Korean East Coast (Waska et al., 2008), with concentrations of (n = 10) 0.9 ± 0.6 ng/g in muscle, 2.4 ± 4.1 ng/g in stomach, 0.9 ± 0.1 ng/g in gills and 3.4 ± 2.5 ng/g in hepatopancreas. These results indicate a moderate Te bioaccumulation, with values in the range of 6 × 103 to 2 × 104 (Waska et al., 2008).
Conclusion and Future Research Priorities
Research on the biogeochemical cycles of the less-studied technology-critical elements (Nb, Ta, Ga, In, Ge, Te) and their ecotoxicology and uptake by biota has been, in general, scarce to date. Therefore the are still important open questions on their environmental behavior and potential impact. The absence of a known biological role for these LSTCEs and the common time-consuming and complicated analytical procedures employed to analyze their trace to ultra-trace concentrations – and the presence of salt normally adds another issue, especially when using spectroscopic techniques – have greatly discouraged marine scientists working on trace elements to include them in their studies. The recent use of these LSTCEs in a number of new technologies – e.g., electronic displays, semiconductors, energy–related technologies or telecommunications technology – has considerably increased the production of these metals at a global scale. Therefore, studies on their biogeochemical behavior and ecotoxicology are expected to increase in the coming years (e.g., Cobelo-García et al., 2015) especially if new analytical procedures, making easier their determination, are developed (e.g., Poehle et al., 2015). Such studies would greatly benefit from the availability of appropriate certified reference materials (in water and biota), which do not exist at present for these elements, in order to guarantee the quality and, therefore, the comparability of the results provided by different authors.
Oceanic profiles have been reported for all the LSTCEs, especially for those that display similar geochemical behavior than ‘key’ oceanic elements. For example, Ga and In have the potential to provide information on the dust input to the surface ocean complementing that obtained using Al, whereas interest in Ge relies on its close similarity with Si as both are subject to cycling by diatoms. From an environmental perspective it is surprising, however, that only very few studies – especially in the recent years – are available on the concentrations and behavior of LSTCEs in estuarine and coastal waters which makes impossible the assessment of their status in environmentally impacted coastal areas. From the available literature, contamination has been observed in waters close to industrialized areas in Japan (In) and Taiwan (Ga), although their concentrations are still below the predicted WQGs for these elements. In marine biota, despite of the fact that concentrations have been reported in several organisms (with the exception of In), information on the factors controlling the LSTCEs uptake (e.g., metal speciation) or their potential to be biomagnified through the food web is mostly missing; accordingly, only two papers reported a study on the trophic transfer – specifically for Ga and Ta – showing a significant biomagnification for Ta but not for Ga.
A scientific effort is therefore encouraged regarding the determination of LSTCEs in different estuarine and coastal areas under varying degrees of anthropogenic influence in order to provide: (i) a better assessment on the impact of the uses of these metals on the concentrations of LSTCEs in such sensitive zones. (ii) Information on the bioavailability of the different chemical forms (e.g., speciation) of the LSTCEs to marine biota, and the factors controlling such speciation (i.e., pH, salinity). (iii) A determination of concentration thresholds for non-lethal endpoints (e.g., stress markers). (iv) A degree of biomagnification through the aquatic food web in order to evaluate potential long-term effects.
Author Contributions
AR-F and PN made the bibliographic search and the first draft of the manuscript. JS-E and AC-G critically revised the manuscript. AC-G made the final revision and English editing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was partially funded by the Xunta de Galicia (ref. IN607A 2016/11).
Acknowledgments
We acknowledge the support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Footnotes
References
Agusa, T., Kunito, T., Yasunaga, G., Iwata, H., Subramanian, A., Ismail, A., et al. (2005). Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Mar. Pollut. Bull. 51, 896–911. doi: 10.1016/j.marpolbul.2005.06.007
Alibo, D. S., Nozaki, Y., and Jeandel, C. (1999). Indium and yttrium in North Atlantic and Mediterranean waters : comparison to the Pacific data. Geochim. Cosmochim. Acta 63, 1991–1999. doi: 10.1016/S0016-7037(99)00080-0
Amakawa, H., Alibo, D. S., and Nozaki, Y. (1996). Indium concentration in Pacific seawater. Geophys. Res. Lett. 23, 2473–2476. doi: 10.1029/96gl02300
Andreae, M. O., Byrd, J. T., and Froelich, P. N. (1983). Arsenic, antimony, germanium and tin in the Tejo Estuary, Portugal: modelling a polluted estuary. Environ. Sci. Technol. 17, 731–737. doi: 10.1021/es00118a008
Astrom, M. E., Peltola, P., Virtasalo, J. J., Kotilainen, A. T., and Salminen, R. (2008). Niobium in boreal stream waters and brackish-water sediments. Geochem. Exp. Environ. Anal. 8, 139–148. doi: 10.1144/1467-7873/07-155
Belzile, N., and Chen, Y. (2015). Tellurium in the environment : a critical review focused on natural waters, soils, sediments and airborne particles. Appl. Geochem. 63, 83–92. doi: 10.1016/j.apgeochem.2015.07.002
Biver, M., Quentel, F., and Filella, M. (2015). Direct determination of tellurium and its redox speciation at the low nanogram level in natural waters by catalytic cathodic stripping voltammetry. Talanta 144, 1007–1013. doi: 10.1016/j.talanta.2015.07.010
Byrne, R. H., Kump, L. R., and Cantrell, K. J. (1988). The influence of pH and temperature on trace metal speciation in seawater. Mar. Chem. 25, 163–181. doi: 10.1016/0304-4203(88)90062-x
Campbell, L. M., Norstrom, R. J., Hobson, K. A., Muir, D. C. G., Backus, S., and Fisk, A. T. (2005). Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Sci. Total Environ. 35, 247–263. doi: 10.1016/j.scitotenv.2005.02.043
Chebotina, M. J., Polyakov, E. V., Guseva, V. P., Khlebnikov, N. A., and Surikov, V. T. (2011). The geochemical role of phyto- and zooplankton in the extraction of chemical elements from water. Doklady Earth Sci. 439, 1138–1140. doi: 10.1134/s1028334x11080113
Cobelo-García, A., Filella, M., Croot, P., Frazzoli, C., Du Laing, G., Ospina-Alvarez, N., et al. (2015). COST action TD1407: network on technology-critical elements (NOTICE)—from environmental processes to human health threats. Environ. Sci. Pollut. R. 22, 15188–15194. doi: 10.1007/s11356-015-5221-0
Costas-Rodríguez, M., Lavilla, I., and Bendicho, C. (2010). Classification of cultivated mussels from Galicia (Northwest Spain) with European protected designation of origin using trace element fingerprint and chemometric analysis. Anal. Chim. Acta 664, 121–128. doi: 10.1016/j.aca.2010.03.003
Di Bella, G., Lo Turco, V., Potorti, A. G., Rando, R., Licata, P., and Dugo, G. (2013). Statistical analysis of heavy metals in Cerastoderma edule glaucum and Venerupis aurea laeta from Ganzirri Lake, Messina (Italy). Environ. Monit. Assess. 185, 7517–7525. doi: 10.1007/s10661-013-3116-4
Ellwood, M. J., and Maher, W. A. (2003). Germanium cycling in the waters across a frontal zone: the Chatham Rise. N. Z. Mar. Chem. 80, 145–159. doi: 10.1016/s0304-4203(02)00115-9
Engström, E., Stenberg, A., Senioukh, S., Edelbro, R., Baxter, D. C., and Rodushkin, I. (2004). Multi-elemental characterization of soft biological tissues by inductively coupled plasma-sector field mass spectrometry. Anal. Chim. Acta 521, 123–135. doi: 10.1016/j.aca.2004.06.030
Espejo, W., Kitamura, D., Kidd, K. A., Celis, J. E., Kashiwada, S., Galbán-Malagón, C., et al. (2018). Biomagnification of tantalum through diverse aquatic food webs. Environ. Sci. Technol. Lett. 5, 196–201. doi: 10.1021/acs.estlett.8b00051
Filella, M. (2017). Tantalum in the environment. Earth Sci. Rev. 173, 122–140. doi: 10.1016/j.earscirev.2017.07.002
Filella, M., Reimann, C., Biver, M., Rodushkin, I., and Rodushkina, K. (2019). Tellurium in the environment: current knowledge and identification of gaps. Environ. Chem. 16, 215–228. doi: 10.1071/EN18229
Filella, M., and Rodríguez-Murillo, J. C. (2017). Less-studied TCE: are their environmental concentrations increasing due to their use in new technologies? Chemosphere 182, 605–616. doi: 10.1016/j.chemosphere.2017.05.024
Filella, M., and Rodushkin, I. (2018). A concise guide for the determination of less-studied technology-critical elements (Nb, Ta, Ga, In, Ge, Te) by inductively coupled plasma mass spectrometry in environmental samples. Spectrochim. Acta Part B Atom. Spect. 141, 80–84. doi: 10.1016/j.sab.2018.01.004
Firdaus, M. L., Mashio, A. S., Obata, H., McAlister, J. A., and Orians, K. J. (2018). Distribution of zirconium, hafnium, niobium and tantalum in the North Atlantic Ocean, northeastern Indian Ocean and its adjacent seas. Deep Sea Res. Part I 140, 128–135. doi: 10.1016/j.dsr.2018.08.008
Firdaus, M. L., Minami, T., Norisuye, K., and Sohrin, Y. (2011). Strong elemental fractionation of Zr – Hf and Nb – Ta across the Pacific Ocean. Nat. Geosci. 4, 227–230. doi: 10.1038/ngeo1114
Firdaus, M. L., Norisuye, K., Nakagawa, Y., Nakatsuka, S., and Sohrin, Y. (2008). Dissolved and labile particulate Zr, Hf, Nb, Ta, Mo and W in the Western North Pacific Ocean. J. Oceanogr. 64, 247–257. doi: 10.1007/s10872-008-0019-z
Froelich, P. N., Hambrick, G. A., Kaul, L. W., Byrd, J. T., and Lecointe, O. (1985a). Geochemical behavior of inorganic germanium in an unperturbed estuary. Geochim. Cosmochim. Acta 49, 519–524. doi: 10.1016/0016-7037(85)90043-2
Froelich, P. N., Kaul, L. W., Byrd, J. T., Andreae, M. O., and Roe, K. K. (1985b). Arsenic, barium, germanium, tin, dimethylsulfide and nutrient biogeochemistry in Charlotte Harbor, Florida, a phosphorous-enriched estuary. Estuar. Coast. Shelf Sci. 20, 239–264. doi: 10.1016/0272-7714(85)90041-1
Froelich, P. N., Mortlock, R. A., and Shemesh, A. (1989). Inorganic germanium and silica in the Indian Ocean: biological fractionation during (Ge/Si)opal formation. Glob. Biogeochem. Cycle 3, 79–88. doi: 10.1029/gb003i001p00079
Gil-Díaz, T., Schäfer, J., Dutruch, L., Bossy, C., Pougnet, F., Abdou, M., et al. (2019). Tellurium behaviour in a major European fluvial-estuarine system (Gironde, France): fluxes, solid/liquid partitioning and bioaccumulation in wild oysters. Environ. Chem. 16, 229–242. doi: 10.1071/EN18226
Guérin, T., Chekri, R., Vastel, C., Sirot, V., Volatier, J.-L., Leblanc, J.-C., et al. (2011). Determination of 20 trace elements in fish and other seafood from the French market. Food Chem. 127, 934–942. doi: 10.1016/j.foodchem.2011.01.061
Höll, R., Kling, M., and Schroll, E. (2007). Metallogenesis of germanium-A review. Ore. Geol. Rev. 30, 145–180. doi: 10.1016/j.oregeorev.2005.07.034
Hsu, S. C., Hsieh, H. L., Chen, C. P., Tseng, C. M., Huang, S. C., Huang, C. H., et al. (2011). Tungsten and other heavy metal contamination in aquatic environments receiving wastewater from semiconductor manufacturing. J. Hazard. Mater. 18, 193–202. doi: 10.1016/j.jhazmat.2011.02.020
Huang, C., and Hu, B. (2008). Speciation of inorganic tellurium from seawater by ICP-MS following magnetic SPE separation and preconcentration. J. Sep. Sci. 31, 760–767. doi: 10.1002/jssc.200700405
Hui, H., Niu, Y., Zhidan, Z., Huixin, H., and Dicheng, Z. (2011). On the enigma of Nb-Ta and Zr-Hf fractionation - a critical review. J. Earth Sci. 22, 52–66. doi: 10.1007/s12583-011-0157-x
Krishna, M. V. B., and Arunachalam, J. (2004). Ultrasound-assisted extraction procedure for the fast estimation of major, minor and trace elements in lichen and mussel samples by ICP-MS and ICP-AES. Anal. Chim. Acta 522, 179–187. doi: 10.1016/j.aca.2004.07.006
Kunito, T., Nakamura, S., Ikemoto, T., Anan, Y., Kubota, R., Tanabe, S., et al. (2004). Concentration and subcellular distribution of trace elements in liver of small cetaceans incidentally caught along the Brazilian coast. Mar. Pollut. Bull. 49, 574–587. doi: 10.1016/j.marpolbul.2004.03.009
Lee, D. S., and Edmond, J. M. (1985). Tellurium species in seawater. Nature 313, 782–785. doi: 10.1038/313782a0
Lewis, B. L., Andreae, M. O., and Froelich, P. N. (1989). Sources and sinks of methylgermanium in natural waters. Mar. Chem. 27, 179–200. doi: 10.1016/0304-4203(89)90047-9
Lewis, B. L., Andreae, M. O., Froelich, P. N., and Mortlock, R. A. (1988). A review of the biogeochemistry of germanium in natural waters. Sci. Total Environ. 73, 107–120. doi: 10.1016/0048-9697(88)90191-x
Lokanc, M., Eggert, R., and Redlinger, M. (2015). The Availability of Indium: the Present, Medium Term, and Long Term. Denver, CO: National Renewable Energy Laboratory.
McAlister, J., and Orians, K. (2012). Calculation of river-seawater endmembers and differential trace metal scavenging in the Columbia River plume. Estuar. Coast. Shelf Sci. 99, 31–41. doi: 10.1016/j.ecss.2011.12.013
Miyazaki, A., Kimura, A., and Tao, H. (2012). Distribution of indium, thallium and bismuth in the environmental water of Japan. Bull. Environ. Contam. Toxicol. 89, 1211–1215. doi: 10.1007/s00128-012-0851-0
Mu, Y., Wu, F., Chen, C., Liu, Y., Zhao, X., and Liao, H. (2014). Predicting criteria continuous concentrations of 34 metals or metalloids by use of quantitative ion character-activity relationships-species sensitivity distributions (QUICAR-SSD) model. Environ. Pollut. 188, 50–55. doi: 10.1016/j.envpol.2014.01.011
Niu, Y. (2012). Earth processes cause Zr-Hf and Nb-Ta fractionations, but why and how? RSC Adv. 2, 3587–3591.
Nolan, C., Whitehead, N., and Teyssie, J. (1991). Tellurium speciation in seawater and accumulation by marine phytoplankton and Crustaceans. J. Environ. Radioactiv. 13, 217–233. doi: 10.1016/0265-931x(91)90062-k
Nozaki, Y., Lerche, D., Alibo, D. S., and Snidvongs, A. (2000a). The estuarine biogeochemistry of rare earth elements and indium in the Chao Phraya River, Thailand. Geochim. Cosmochim. Acta 64, 3983–3994. doi: 10.1016/s0016-7037(00)00473-7
Nozaki, Y., Lerche, D., Alibo, D. S., and Tsutsumi, M. (2000b). Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and In. Geochim. Cosmochim. Acta 64, 3975–3982. doi: 10.1016/s0016-7037(00)00472-5
Obata, H., Alibo, D. S., and Nozaki, Y. (2007). Dissolved aluminum, indium, and cerium in the Sea of Japan and the Sea of Okhotsk : comparison to the marginal seas of the western North Pacific. J. Geophys. Res. 112, 1–10.
Obata, H., Nozaki, Y., Alibo, D. S., and Yamamoto, Y. (2004). Dissolved Al, In, and Ce in the eastern Indian Ocean and the Southeast Asian Seas in comparison with the radionuclides 210 Pb and 210 Po. Geochim. Cosmochim. Acta 68, 1035–1048. doi: 10.1016/j.gca.2003.07.021
Orians, K. J., and Bruland, K. W. (1988). The marine geochemistry of dissolved gallium: a comparison with dissolved aluminium. Geochim. Cosmochim. Acta 52, 2955–2962. doi: 10.1016/0016-7037(88)90160-3
Poehle, S., Schmidt, K., and Koschinsky, A. (2015). Determinatin of Ti, Zr, Nb, V, W and Mo in seawater by a new online-preconcentration method and subsequent ICP-MS analysis. Deep Sea Res. I 98, 83–93. doi: 10.1016/j.dsr.2014.11.014
Qie, Y., Chen, C., Guo, F., Mu, Y., Sun, F., Wang, H., et al. (2017). Predicting criteria continuous concentrations of metals or metalloids for protecting marine life by use of quantitative ion characteristic-activity relationships-species sensitivity distributions (QUICAR-SSD). Mar. Pollut. Bull. 124, 639–644. doi: 10.1016/j.marpolbul.2017.02.055
Ramos-Ruiz, A., Zeng, C., Sierra-Alvarez, R., Teixeira, L. H., and Field, J. A. (2016). Microbial toxicity of ionic species leached from the II-VI semiconductor materials, cadmium telluride (CdTe) and cadmium selenide (CdSe). Chemosphere 162, 131–138. doi: 10.1016/j.chemosphere.2016.07.081
Rongguo, C., Juan, G., Liwen, Y., Huy, D., and Liedtke, M. (2016). Supply and Demand of Lithium and Gallium. Hannover: Federal Institute for Geosciences and Natural Resources.
Rudnick, R. L., and Gao, S. (2003). Composition of the continental crust. Treat. Geochem. 1, 1–64. doi: 10.1016/b0-08-043751-6/03016-4
Sánchez-Rodríguez, I., Huerta-Díaz, M. A., Choumiline, E., Holguín-Quiñones, O., and Zertuche-González, J. A. (2001). Elemental concentrations in different species of seaweeds from Loreto Bay, Baja California Sur, Mexico: implications for the geochemical control of metals in algal tissue. Environ. Pollut. 114, 145–160. doi: 10.1016/s0269-7491(00)00223-2
Santosa, S. J., Wada, S., Mokudai, H., and Tanaka, S. (1997). The contrasting behaviour of arsenic and germanium species in seawater. App. Organometal. Chem. 11, 403–414. doi: 10.1002/(sici)1099-0739(199705)11:5<403::aid-aoc596>3.3.co;2-w
Shiller, A. M., and Bairamadgi, G. R. (2006). Dissolved gallium in the northwest Pacific and the south and central Atlantic Oceans: implications for aeolian Fe input and a reconsideration of profiles. Geochem. Geophys. Geosyst. 7, 1–14. doi: 10.1029/2005GC001118
Sohrin, Y., Fujishima, Y., Ueda, K., Akiyama, S., Mori, K., Hasegawa, H., et al. (1998). Dissolved niobium and tantalum in the North Pacific. Geophys. Res. Lett. 25, 999–1002. doi: 10.1029/98gl00646
Sutton, J., Ellwood, M. J., Maher, W. A., and Croot, P. L. (2010). Oceanic distribution of inorganic germanium relative to silicon: germanium discrimination by diatoms. Glob. Biogeochem. Cycl. 24:GB2017. doi: 10.1029/2009GB003689
USGS (2018). Selenium and Tellurium. Statistics and Information. Available at: https://minerals.usgs.gov/minerals/pubs/commodity/selenium (accessed June, 2019).
van Dam, J. W., Trenfield, M. A., Streten, C., Harford, A. J., Parry, D., and van Dam, R. A. (2018). Water quality guideline values for aluminium, gallium and molybdenum in marine environments. Environ. Sci. Pollut. Res. 25, 26592–26602. doi: 10.1007/s11356-018-2702-y
Waska, H., Kim, S., Kim, G., Kang, M. R., and Kim, G. B. (2008). Distribution patterns of chalcogens (S, Se, Te, and 210Po) in various tissues of a squid, Todarodes pacificus. Sci. Total Environ. 392, 218–224. doi: 10.1016/j.scitotenv.2007.12.003
Wu, X., Song, J., and Li, X. (2014). Occurrence and distribution of dissolved tellurium in Changjiang River estuary. Chin. J. Oceanol. Limnol. 32, 444–454. doi: 10.1007/s00343-014-3161-z
Yoon, B. M., Shim, S. C., Pyun, H. C., and Lee, D. S. (1990). Hydride generation atomic absorption determination of tellurium species in environmental samples with in situ concentration in a graphite furnace. Anal. Sci. 6, 561–566. doi: 10.2116/analsci.6.561
Keywords: technology critical elements, seawater, organisms, concentrations, bioaccumulation
Citation: Romero-Freire A, Santos-Echeandía J, Neira P and Cobelo-García A (2019) Less-Studied Technology-Critical Elements (Nb, Ta, Ga, In, Ge, Te) in the Marine Environment: Review on Their Concentrations in Water and Organisms. Front. Mar. Sci. 6:532. doi: 10.3389/fmars.2019.00532
Received: 30 January 2019; Accepted: 13 August 2019;
Published: 11 September 2019.
Edited by:
Ketil Hylland, University of Oslo, NorwayCopyright © 2019 Romero-Freire, Santos-Echeandía, Neira and Cobelo-García. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Cobelo-García, acobelo@iim.csic.es
 Ana Romero-Freire
Ana Romero-Freire Juan Santos-Echeandía
Juan Santos-Echeandía Patricia Neira
Patricia Neira Antonio Cobelo-García
Antonio Cobelo-García