Life cycle assessment of auto-tropically cultivated economic microalgae for final products such as food, total fatty acids, and bio-oil
- 1School of Mechanical Engineering, Xi’an Shiyou University, Xi’an, China
- 2Shenzhen Key Laboratory of Marine Bioresource and Eco−Environmental Science, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen, China
- 3Shenzhen Engineering Laboratory for Marine Algal Biotechnology, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen, China
- 4Guangdong Provincial Key Laboratory for Plant Epigenetics, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen, China
Life cycle assessment (LCA) is a powerful tool to evaluate environmentally sustainable production or consumption of various goods or services. Microalgae are single-celled green factories and good resources of biofuels, bioactive products, food ingredients, and degradable biomaterials. Currently, microalgae are also valuable for mitigating elevated greenhouse gases like CO2 levels and treatment of wastewater. LCA evaluation was limited and separated, majorly in microalgal biofuels and heterotrophic cultivation. Comparative LCA for different final algal products such as algal powder, bio-oil, total fatty acid, and residue recycling is still limited, especially autotrophic algal cultivation for products other than bio-oils and biofuels. Thus, we chose several autotrophic cultivated microalgae and made a comparative LCA among these selected species and a detailed step-by-step production in Chlorella sp. Results indicated that we could significantly reduce the production cost and lower environmental impacts by selecting algal species and final products, optimizing methods for algal cultivation, biomass separation, and drying process, and land selection plus electricity renewable energy, together with thermal power plants nearby for CO2 or flu gas. It shed light on the insight of microalgal consumption selection under current international requirements and challenges for carbon sequestration.
Introduction
Carbon dioxide CO2 is the most commonly man-produced greenhouse gas. Carbon sequestration is the process of capturing and storing atmospheric CO2, as a method to reduce the amount of CO2 in the atmosphere to reduce elevated global climate change (https://www.usgs.gov/). Now it is a life-or-death race to improve carbon capture (acs.org). Microalgae could be one of the potential climate change-mitigating biological agents available with a green approach for sustainable development (Patidar and Mishra, 2017) and treatment of wastewater (Khan et al., 2018).
Microalgae are sources of biofuels, bioactive medicinal products, food ingredients, and degradable biomaterials. The main bioactive substances in microalgae include proteins, lipids, polysaccharides, pigments, and vitamins. Microalgae are also used as good sustainable resources in value-added products (Matos, 2019; Dolganyuk et al., 2020; Schade et al., 2020; Amorim et al., 2021). The most extensive massive algal cultivation focused on algal biofuels (Campbell et al., 2011; Collet et al., 2011; Clarens and Colosi, 2013; Adesanya et al., 2014; Chiaramonti et al., 2015; Hosseinzadeh-Bandbafha et al., 2020). High protein contents were already detected and consumed in several economic microalgal species (e.g., 55%–70% for Spirulina platensis and 42%–55% for Chlorella vulgaris per dry matter) as summarized by Matos (2019). The global potential for using microalgal biomass as a source of protein is recognized in history for microalgae’s safe and essential nutrients (Matos, 2019; Amorim et al., 2021). Recently, specific algal products, were significantly marked, dietary supplementary such as fucoxanthin (Khoo et al., 2021), microalgae-based polyhydroxyalkanoates, bioplastics (Beckstrom et al., 2020; Chong et al., 2021a; Chong et al., 2022) using food waste hydrolysate and wastewater (Chong et al., 2021b), and various bioenergy production (Chia et al., 2022).
Life-cycle assessment (LCA) is a powerful tool used to evaluate the environmentally sustainable production and consumption of various goods and services. Some LCAs were already applied in the field of microalgal biofuels (Moody et al., 2014; Fortier et al., 2014; Bradley et al., 2015; Dutta et al., 2016; Gnansounou and Kenthorai Raman, 2016; Guo et al., 2016; Kumar et al., 2017; Foteinis et al., 2018; Shi et al., 2019; Depra et al., 2020; Hosseinzadeh-Bandbafha et al., 2020; Mediboyina et al., 2020; Schneider et al., 2018; Chen and Quinn, 2021; Somers et al., 2021; Guiton et al., 2022). Inexpensive petroleum fuels make algal biofuels woefully uncompetitive, and alternative coproducts from algae provide the potential to more effectively apply biomass in terms of economic viability (Beckstrom et al., 2020).
Microalgal LCA was also conducted in some microalgal species, such as bioplastic feedstock production from Scenedesmus acutus (UTEX B72) (Beckstrom et al., 2020), heterotrophic fermentation (Lu et al., 2021), astaxanthin production from Haematococcus pluvialis (Pérez-López et al., 2014; Onorato and Rösch, 2020), non-energy purposes from Phaeodactylum tricornutum (Porcelli et al., 2020), Acutodesmus obliquus (SAG 276-10) bioactive algal extracts (Sandmann et al., 2021), Spirulina tablets (Ye et al., 2018), Tetraselmis chui algal biomass, bio-oil (Grierson et al., 2013), Nannochloropsis oceanica TFA (Gaber et al., 2021), heterotrophic algae omega-3 (Davis et al., 2021), microalgae-based biofertilizer (Castro et al., 2020), and Chlorella vulgaris biorefineries (Bussa et al., 2021). The results indicate that alternative microalgal final products focused on non-biofuel production had the potential to operate both economically and sustainably (Pérez-López et al., 2014; Beckstrom et al., 2020; Castro et al., 2020; Onorato and Rösch, 2020; Porcelli et al., 2020; Bussa et al., 2021; Davis et al., 2021; Gaber et al., 2021; Sandmann et al., 2021).
So far, most microalgal LCAs were focused majorly on biofuels and heterotrophic cultivation. During literature analyses, we noticed the inconsistency of LCAs of microalgal products, caused mainly by the extrapolation of laboratory data, inconsistencies in system boundaries, and differences in production pathway architecture, country standards, and databases. Comparative LCAs for different final microalgal products such as algal powder, bio-oil, total fatty acid, and residue recycling are still somehow limited, especially autotrophic algal cultivation for products other than bio-oils and biofuels. Thus, we chose several autotrophic cultivated microalgae, such as Haematococcus pluvialis, Desmodesmus subspicatus, and Nannochloropsis oceanica, to compare LCAs of selected species and a detailed step-by-step production in Chlorella sp.
Based on this study, the direction of future microalgal mass cultivation and products should focus on reaching lower-energy consumption with more minor environmental damages and human toxicity, which will also save resource use and promote environmental benefits and human living quality.
Methodology
The selection of algal strains and products was mainly based on their relative complete and clear input and output data. We collected some published data majorly based on the GaBi LCA system, including algal powders from Haematococcus (Onorato and Rösch, 2020) and Desmodesmus subspicatus (Schneider et al., 2018), total fatty acid production from Nannochloropsis oceanica (Gaber et al., 2021), and Spirulina tablets (Ye et al., 2018). For a more accurate evaluation and comparison of three different life stages, flocculation, disruption, and production of bio-oil from Chlorella (Lu et al., 2021), values for midpoint environmental impact categories were calculated with eFootPrint based on data collected in China. The process and related information are summarized in Figure 1.
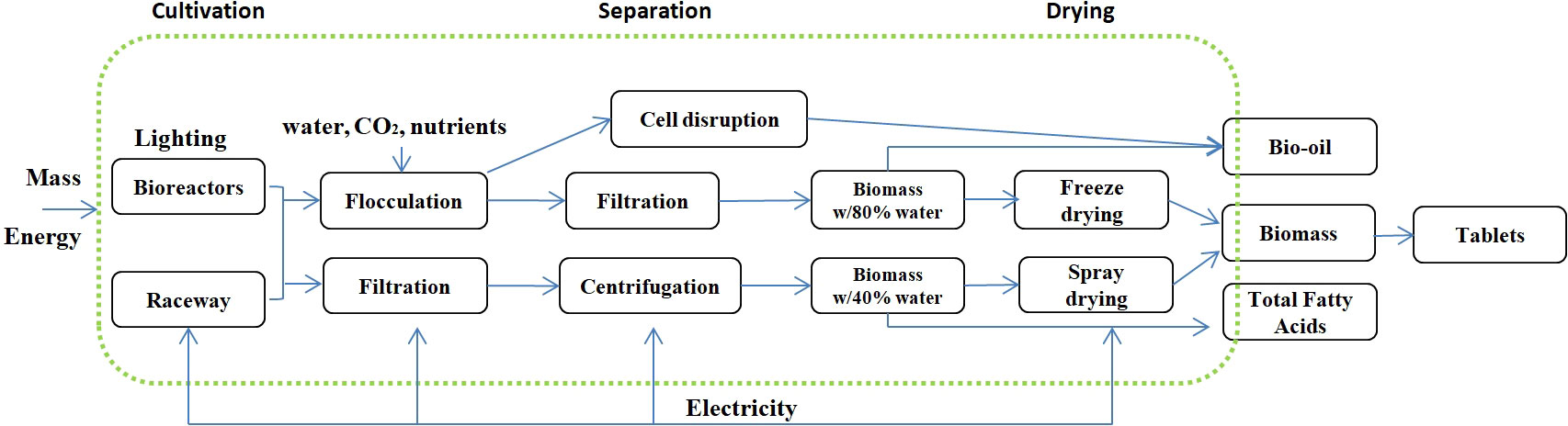
Figure 1 Product system for autotrophic microalgal biomass, bio-oil, total fatty acids, and other products.
The study was carried out using previously described processes (Guo et al., 2021; Ding et al., 2022). Briefly, this is a four-stage life-cycle methodology assessment based on standards developed by the International Organization for Standardization (ISO) for life-cycle assessment as ISO 14040 (2006) and ISO 14044 (2006), including research objectives and scope, inventory analysis, and impact assessment.
Objectives and scope
LCA analyzed the environmental impact of different stages of bio-oil production from algal cultivation to the end of production (algal culture for aquaculture feed, soil bio-stimulator; disrupted cells for a facial cleanser, and bio-oil), which focuses on material consumption, energy use, and waste generated during production.
The function unit is defined as producing 1 kg of products. The actual process and system boundary of products are shown in Figure 1. The system boundary considers all the energy and material input and environmental output related to products from cradle to gate. The system boundary does not include the CO2 fixing process.
LCI analysis
The authors investigated three production data based on previously reported inputs (Lu et al., 2021). According to previous literature, the Chlorella cultivation facilities are modeled from pilot-scale facilities (Lu et al., 2021). The microalgal cells collected from the open pond, coagulation (flocculation, algal slurry as the product), and centrifuging processes were modeled for dewatering. Then, the algae underwent pressure homogenization pretreatment to break cell walls (disruption, cell extracts as the product) and facilitate lipid extraction (bio-oil) (Clarens et al., 2011). The lipid was extracted with a solvent (hexane) process called “wet lipid extraction” with no intensive drying process needed. The input and output parameters are included in the Supplementary information (SI) (Table S1, Lu et al., 2021).
Impact assessment
eFootPrint is an online platform for analyzing LCA data by IKE Environmental Technology Co., Ltd., and contains CLCD and Ecoinvent (Jiao et al., 2019; Mi and Kunliang, 2019). CLCD includes more than 600 LCI datasets for raw materials, chemicals, energy, transportation, and waste treatment (IKE, 2012a; IKE, 2012b). The selected categories are global warming potential GWP (kg CO2 eq), primary energy demand PED (MJ), resource depletion-water WU (kg), acidification potential AP (kg SO2 eq), abiotic depletion ADP (kg antimony eq.), eutrophication potential EP (kg (PO4)3-eq), respiratory inorganics RI (kg PM2.5 eq), ozone layer depletion ODP (kg CFC-11 eq), photochemical oxidant formation POFP (kg NMVOC eq), ionizing radiation IRP (kg U235 eq), eutrophication (ET) ET (CTUe), human toxicity HT-cancer (CTUh), HT-non cancer (CTUh), SO2 (kg), CO2 (kg), NOx (kg), NH3-N (kg), chemical oxygen demand COD (kg), industry water use IWU (kg), global warming potential agriculture GWP-A (kg CO2 eq), nitrogen footprint agriculture NF-A (kg N eq), potential water use agriculture (L H2O eq), and high NOx POCP - photochemical oxidation (summer smog) (kg ethylene-Eq).
Results and discussion
Comparison of three Chlorella products
Originally, we collected as much LCA data as we could for further analyses based on the eFootPrint platform. However, we noticed a significant difference in some inventory impacts and obtained different LCA results. Due to the unavailability of GaBi currently, we chose a simple sample for autotrophic cultivation of Chlorella (Lu et al. (2021) based on Chinese standards on the eFootPrint platform.
The LCA results for flocculation, disruption, and bio-oil from Chlorella production are presented in Figure 2. In general, the environmental impacts of the first two products were less than those of bio-oil production, with the most significant impact of primary energy demand PED and human toxicity HT non-cancer and cancer (Figure 2). The flocculation step had the same impacts as the disruption in all other indexes except for lower primary energy demand PED and human toxicity HT non-cancer and cancer. For flocculation and disruption stages, the algal cultivation caused more than 80% of the total impacts in all categories (as 23.778 kWh electricity, 0 heat MJ per 1 kg), making this stage a critical stage of environmental impacts. For bio-oil production, heat (6.830 MJ per 1 kg, with 0.51 kWh electricity) was the secondary contributor to the total impacts. Thus, algal processing stages like disruption and oil extraction contributed much fewer impacts than the cultivation stage in this case.

Figure 2 Contribution to relevant environmental impact categories for three steps as flocculation, disruption, bio-oil, the production of 1 kg bio-oil the production of 1 kg bio-oil production with Chlorella for the eFootPrintas. Contributions with low (A) and relative high impact values (B), respectively.
For all selected impact categories except for water use WU and NH3-N (Figure 3), their LCA results showed the same trend, i.e., electricity caused 64%~93% of the impacts of all three productions. In contrast, freshwater and polyacrylamide (PAM) contributed to the impacts of WU (93.1%) and NH3-N (79.4%), respectively (Figures 3A, B). For bio-oil production, heat and NaOH contributed more to HT non-cancer and cancer than urea and diammonium phosphate (DAP) in the flocculation and disruption stages.
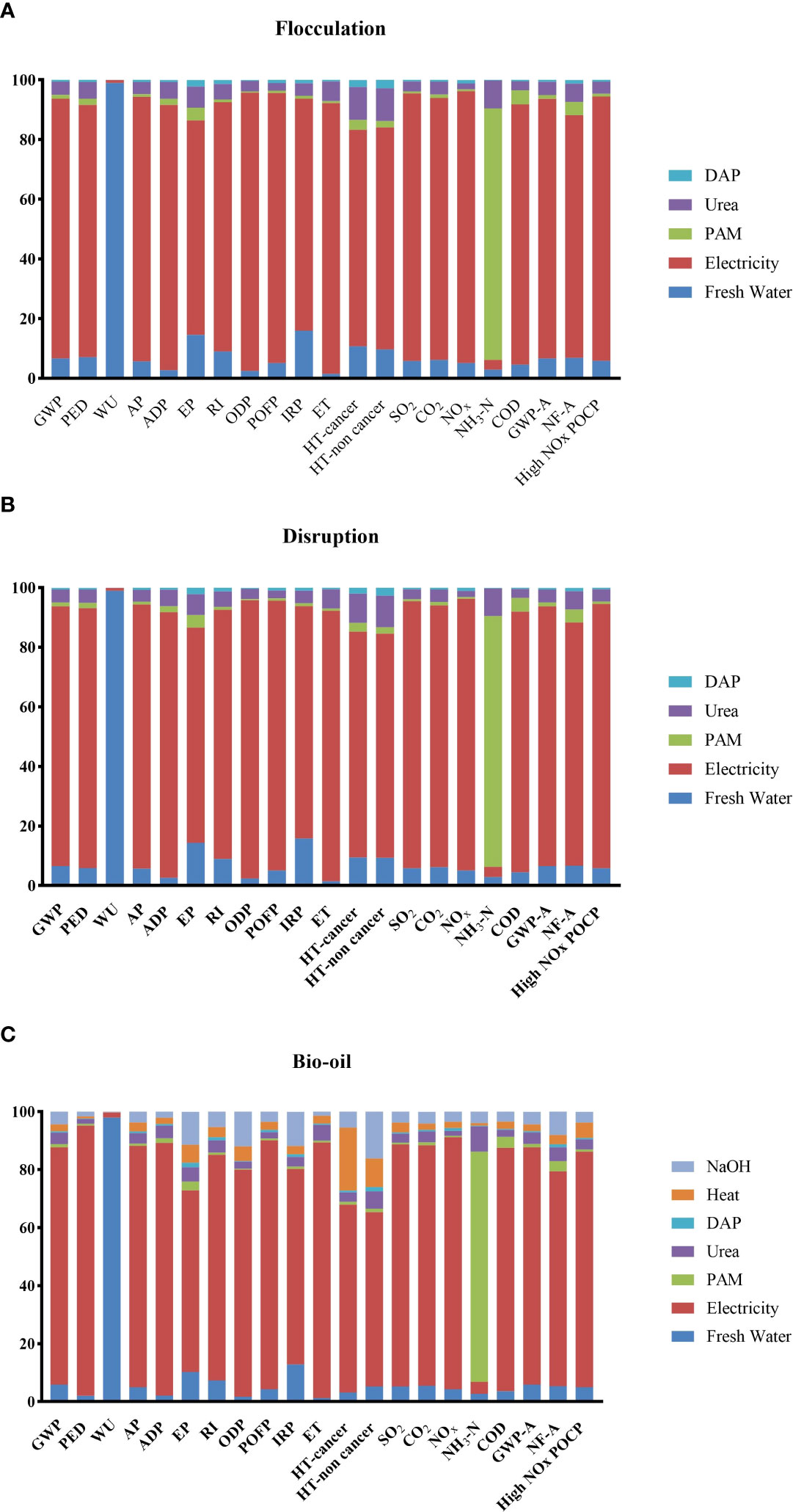
Figure 3 Potential environmental impact for the production steps as 1 kg of flocculation (A), disruption (B), bio-oil, (C) the production of 1 kg bio-oil with Chlorella for the eFootPrint. The total impacts were set as 100% in the figures. Global warming potential GWP (kg CO2 eq), primary energy demand PED (MJ), resource depletion-water WU (kg), acidification potential AP (kg SO2 eq), abiotic depletion ADP (kg antimony eq.), eutrophication potential EP (kg (PO4)3-eq), respiratory inorganics RI (kg PM2.5 eq), ozone layer depletion ODP (kg CFC-11 eq), photochemical oxidant formation POFP (kg NMVOC eq), ionizing radiation IRP (kg U235 eq), eutrophication (ET) ET (CTUe), human toxicity HT-cancer (CTUh), HT-non cancer (CTUh), SO2 (kg), CO2 (kg), NOx (kg), NH3-N (kg), chemical oxygen demand COD (kg), industry water use IWU (kg), global warming potential agriculture GWP-A (kg CO2 eq), nitrogen footprint agriculture NF-A (kg N eq), potential water use-agriculture (L H2O eq), high NOx POCP - photochemical oxidation (summer smog) (kg ethylene-Eq).
Based on LCA results, the three production stages, that is, flocculation, disruption, and bio-oil production, increased 0.59–0.67 kg of CO2 per 1-kg product (Figures 3A–C; Table S1). If the autotrophic cultivation of 1 kg Chlorella can absorb 1.8–2.0 kg CO2, in all three stages in this study, we will have a 1.2–1.6-kg CO2 sequestration.
Comparison of three microalgal products
The potential environmental impact of LCA results in 1-kg algal powders of Haematococcus and Desmodesmus subspicatus and 1 kg of total fatty acid (TFA) from Nannochloropsis oceanica production, which were adapted from the literature and are presented in Figures 4A–C.
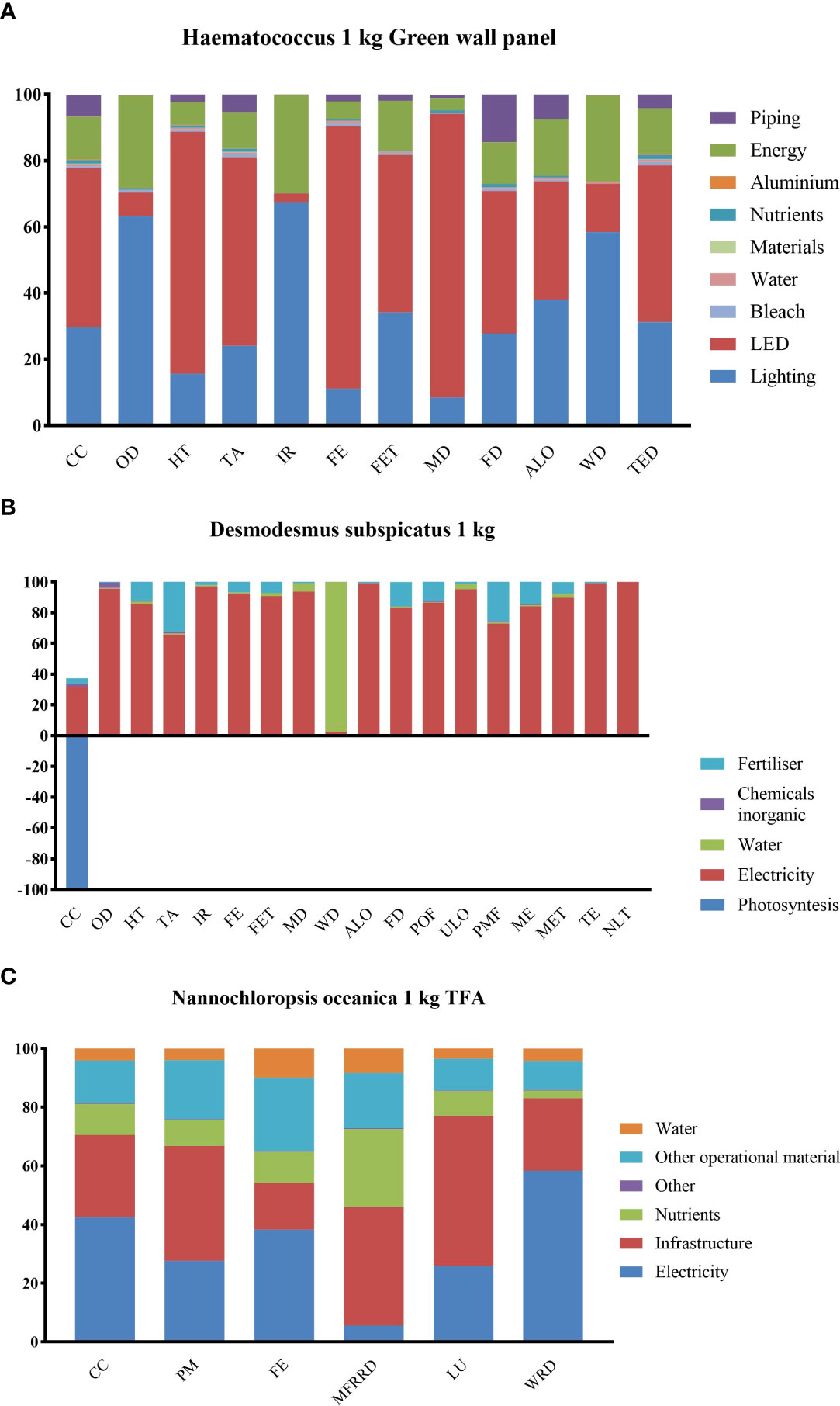
Figure 4 Potential environmental impact for the production of (A) 1 kg of H pluvialis using Green Wall Panel as photobioreactors. Climate change (CC), terrestrial acidification (TA), ionizing radiation (IR), human toxicity (HT), water depletion (WD), terrestrial ecotoxicity (TET), ozone depletion (OD), freshwater eutrophication (FE), freshwater ecotoxicity (FET), metal depletion (MD), fossil depletion (FD), agricultural land occupation (ALO); (B) 1 kg of Desmodesmus subspicatus using NPK culture medium. climate change (CC), ozone depletion (OD), human toxicity (HT), photochemical oxidant formation (POF), particulate matter formation (PMF), ionizing radiation (IR), terrestrial acidification (TA), freshwater eutrophication (FE), marine eutrophication (ME), terrestrial ecotoxicity (TET), freshwater ecotoxicity (FET), marine ecotoxicity (MET), agricultural land occupation (ALO), urban land occupation (ULO), natural land transformation (NLT), water depletion (WD), metal depletion (MD), fossil depletion (FD); (C) 1 kg of TFA from Nannochloropsis oceanica using NPK culture medium. Climate change (CC), particulate matter (PM), freshwater eutrophication (FE), mineral, fossil, and renewable resource depletion (MFRRD), land use, water resource depletion (WSD).
Energy consumption contributed to all scenarios during algal cultivation, separation, and drying to the global warming potential and other catalogs such as ozone depletion, acidification, ionizing radiation, human toxicity, eutrophication, and fossil depletion. For all microalgal products, we selected in this study energy, including electricity, lighting, LED, piping, and other energy for the process, which was classified as the significant impact. Lighting and LED, together with sunlight for outdoor cultivation, are necessary for algal photosynthetic growth. In Haematococcus production, energy impacts (lighting, LED, energy for the process, and piping) accounted for 97.35% of GWP production at the cultivation stage. Moreover, lighting and LED showed 77.78% energy consumption (Figure 4A). Even in 1-kg TFA production from Nannochloropsis, infrastructure was counted for GWP production with electricity still showing 31.94% contribution (Figure 4C). Furthermore, energy or electricity consumption in algal cultivation and production accounted for the greatest contributions to ozone depletion, acidification, ionizing radiation, human toxicity, eutrophication, and fossil depletion.
For the global warming potential catalog, 1 kg of Haematococcus produced 95.34 kg GWP and 1106~4015 kg CO2-equiv for 1-kg astaxanthin production based on different country standards. In comparison, 0.8 kg CO2-equiv was produced for the total fatty acid of Nannochloropsis, and -1.24 kg CO2-equiv was obtained for 1 kg of Desmodesmus (Figure 4B).
Water and nutrients are necessary for algal growth and biomass production. Since autotrophic cultivation depends on light penetration for algal photosynthesis, the cell density of algae growing in raceway ponds or photobioreactors could only maintain on average levels of 0.5–3.0 kg DW/l. Thus, a massive volume of water will be required for algal growth and biomass production, and dewatering or drying will be necessary for some scenarios. In most cases, water is recycled, and thus in LCA results, water itself showed little impact on human health and the lowest for resource damages.
Land and water resources are often the critical limiting factors for the large-scale cultivation of microalgae. When the raceway open pond is adopted to cultivate microalgae, large-scale cultivation could obtain high economic benefits compared to other closed photobioreactors. Therefore, it is necessary to occupy a particular land area (Zhao and Huang, 2021). In one of our samples, production of 1 kg TFA from Nannochloropsis, the infrastructure, electricity, and other operational materials accounted for 80.68% of an average 31.15 land use (kg C deficit) (Gaber et al., 2021). For a raceway pond, a total of 8,000-l volume of culture required a 32-m2 cultivation area to produce 9–12 kg algae in 10 days with agriculture land occupation of 1.4 m2a per 1 kg of algal powder (Schneider et al., 2018).
Different microalgal species and culture medium
Compared with water, more environmental impacts were observed for nutrients for algal production. In the production of Desmodesmus, nutrients (fertilizers with N, P, K) have significant impacts on acidification (32.27%), PM formation (25.5%), eutrophication (14.34%), food depletion (15.9%), human toxicity (12.35%), POF (12.4%), and ecotoxicity (7.2%). Since 1-kg production of NaNO3 requires 26.6 MJ of energy, any reduction in the nutrient use or alternative chemicals would directly affect the energy demand and subsequently the environmental performance of the process (Schneider et al., 2018).
It is well known that nutrients such as urea, glucose, and NH4NO3 in the culture medium can contribute to environmental impacts such as acidification, eutrophication, and ecotoxicity (Ye et al., 2018; Guo, 2019; Nawkarkar et al., 2019; Lu et al., 2021). Also, nutrient input is the main factor leading to the change in total environmental impact when the power input per bio-oil production unit decreases under heterotrophic cultivation using Chlorella (Lu et al., 2021). Such significant differences between culture medium, growth, and lipid productivity indeed contribute to different environmental impacts and human health damages and benefits. An LCA was conducted on Chlorella species producing biodiesel and remediating wastewater (WW) compared with BG11 and TAP as culture medium, respectively, showing the highest lipid and biomass productivity and almost twice fatty acid methyl ester (FAME) in WW compared to BG11 and TAP media (Nawkarkar et al., 2019).
In a previous Spirulina LCA, the urea used in algal cultivation contributed to 69% of the total impact because the acidification of urea production was much higher than in other industries. The sodium bicarbonate caused 13.5% of the acidification potential, and electricity use caused the other 10% (Ye et al., 2018).
For the 1-t Chlorella production for biofuels (Guo, 2019), the most output catalog is CO2, 6.68 × 103 kg, with other greenhouse gases such as CH4, SO2, NOx, CO, and NMVOC at the 19–1.14-kg levels. Among all stages, algal cultivation contributed the highest CO2 (55.23%), and algal harvesting counted the second (30.84%). LCA results showed that GWP at algal cultivation was -5.45 × 103 kg CO2-equiv, since green algal cells absorbed CO2 for photosynthesis and growth, and the amount of CO2 absorption was more significant than emission during electricity and other energy used for productions (+3.26 × 103 kg CO2-equiv). In the end, the production of algal biofuels is a process of CO2 sequestration (Guo, 2019).
COD, as the most significant output into the water, was majorly demised at the stage of algal harvesting, 51.61% of the total 3.41 kg. Thus, based on the environmental impacts, the stages with the most contribution were algal harvesting and cultivation, while environmental pollution caused by bio-oil extraction and esterification was the lowest (Guo, 2019). The AP catalog contribution list consisted of harvesting (54%), cultivation (23%), and bio-oil (19%) extraction. EP contributed to algal cultivation and harvesting (Guo, 2019).
In Desmodesmus subspicatus biomass production in a raceway pond (8,000 l), electricity input during algal cultivation was 360.8 kWh, with 8.96 and 5.95 for flocculation, 25.5 for centrifugation, and 26.4–52.8 for oven drying (Schneider et al., 2018). During the process of algal biofuels, the electricity contributed the most of GWP, AP, EP, and POCP (photochemical oxidation); ADP fossil and urea contributed to ADP, fossil, and GWP; and electricity for algal drying caused AP (Guo, 2019).
In summary, autotrophic algal cultivation for biofuels reduced CO2 and caused a negative increase in greenhouse gases; however, a huge amount of electricity and fertilizers was used and caused the depletion of fossil oils, acidation, eutrophication, and POCP (Guo, 2019). We could calculate the CO2 fixed from total algal biomass obtained by autotrophic cultivation, considering that 1 kg of algal biomass corresponds to approximately 1.83 kg of fixed CO2 (Pires, 2017). In our system, we used atmospheric CO2, and therefore, the fixation was 22 kg CO2 (−14.87 kg CO2 eq total) for production in NPK solution. The effect in the climate change category made the use of a culture medium named as NPK solution more interesting because there is 23% less kg CO2 eq in the atmosphere in this case (Schneider et al., 2018).
Wastewater (WW) has organic and inorganic supplements required for algal growth. The coupling of microalgae with WW is an effective way of waste remediation and cost-effective microalgal biofuel production (Bhatt et al., 2014). Nawkarkar et al. (2019) proposed that WW is better for microalgal products using Chlorella species. In another previous study, the long-term impacts were lower when the Desmodesmus cultures were grown in WW than other culture media in a raceway pond (Schneider et al., 2018).
Many microalgal species, such as Scenedesmus sp., Dunaliella tertiolecta, Pleurochrysis carterae, Botryococcus braunii, Chlorella sorokiniana, and C. vulgaris, have shown potential for remediation of WW and have been extensively studied (Podder and Majumder, 2016; Asadi et al., 2019; Nawkarkar et al., 2019; Chen et al., 2020; Ye et al., 2020; Aggarwal et al., 2021; Mohseni et al., 2021). They showed different growth patterns and various qualities of FAME for biofuels under different WW and culture conditions (Nawkarkar et al., 2019).
Process and products
When single-celled algal cells are agglomerated, the separation or dewatering is more efficient. Flocculation has been used successfully for this purpose. After separation, the supernatant was returned to the process, and the biomass was dried (Schneider et al., 2018). Compared with separation of the biomass by electroflotation (Al or Fe), NaOH flocculation has higher values mainly for climate change and human toxicity, due to the NaOH used (Schneider et al., 2018). The biomass can also be separated directly by culture medium centrifugation, with much more time and energy consumption: 25.5 kWh for centrifuge vs. 5.95~8.96 kWh for Fe or Al flocculations in Desmodesmus subspicatus 8,000-l raceway cultivation (Schneider et al., 2018), and 1.0 kg MJ-1 for centrifuge vs. 0.01–0.1 kg MJ-1 for FeCl3 flocculation of Scenedesmus dimorphus in a raceway pond (Mediboyina et al., 2020). Similarly, moderate electricity requirements were needed for the centrifuge harvest of Haematococcus in different photobioreactors (Onorato and Rösch, 2020). Centrifugation has no environmental impacts related to the use of chemical components of NaOH; however, the cost of energy consumption is higher for centrifugation (Schneider et al., 2018; Mediboyina et al., 2020; Onorato and Rösch, 2020). Thus, electro-flotation could be a better choice for algal separation than chemicals such as aluminum sulfate or NaOH flocculation. Furthermore, it was reported that flocculation followed by centrifugation has smaller impacts than centrifugation alone for all of the environmental impact categories, since much lower water contents are left for centrifuge after flocculation (Collotta et al., 2017). Flocculation combined with centrifuge could probably be an energy-saving process for single-celled algal dewatering.
Due to energy consumption, biomass drying still contributes to many environmental impacts. The biomass was obtained with ~40% and ~80% water after centrifugation and filtration, respectively. Different water percentages of biomass result in different drying times and energy consumption in the volume of water. In Haematococcus, downstream processes such as filtration, centrifugation, and spray drying with 1.5%–10%, 10%–20%, and 90%–95% of solid concentrations, respectively, cost 640, 219, and 92,000 kWh/year in flat panel airlift (FPA) bioreactors (Onorato and Rösch, 2020). Drying is environmentally friendly, but it still consumes a huge amount of electricity. For different final algal products, the drying process can be omitted. For example, lipid extraction from algal slurry resulted in a 43.83% improvement in the life cycle of algal biodiesel compared to the extraction of lipids from dried algae (Jian et al., 2015).
The life cycle assessment of Spirulina tablets revealed that edible algal production has two to five times high environmental impacts than algal biofuels due to the high contents of nutrients used in the algal cultivation stage and electricity use for various downstream processes. To decrease the economic cost and environmental impacts of algal food products, several strategies such as strain selections, photo-bioreactor design improvements, use of alternative renewable energy, and recycling of CO2 could be considered and adapted (Ye et al., 2018).
Due to the energy and material costs during algal cultivation and product production, current algae-based commercial products are still relatively higher than other products in the market. The ability for algae-based products to compete effectively at scale in new and existing markets, such as food, bio-oil, and bio-materials, will inevitably be based on price but also comparable reductions in environmental impact (Grierson et al., 2013). For example, using algal omega-3 DHA in feed can reduce pressure on fish requirements and marine resources (Davis et al., 2021). Optimizing protein production using C. pyrenoidosa on wastewater could significantly reduce environmental impact by ~4.5 times as one of the most sustainable alternative protein sources against pork and beef (Smetana et al., 2017). In all, algae-based bioproducts will be more environmentally friendly.
Land selection and CO2 resources
Although algae are not competing with traditional terrestrial crops for valuable agricultural land, algal autotrophic massive cultivation on a large scale, i.e., 1,000 m3 or more, still has limitations of land as a resource (Quinn and Davis, 2015). The annual average lipid productivity modeled in Pate et al. (2011) and Quinn et al. (2011) was 19.6, 4.64, and 18 m3 ha-1 year-1, respectively. Differences in land availability and geographic restriction assumptions led to differences in land availability results, 19, 43, and 75 million hectares reported (Pate et al., 2011; Quinn et al., 2011; and Quinn and Davis, 2015), respectively. If we consider algal biomass and other bioproducts for massive cultivation, we could refer to the model based on the photosynthetic efficiency and fixed lipid content (Quinn et al., 2011).
In this study, air containing CO2 or food-grade CO2 was used for CO2 resources (Lu et al., 2021). Carbon dioxide represents an essential nutrient, but many studies underestimate the CO2 transport and delivery cost. Challenges associated with the economical delivery and utilization of CO2 have typically been ignored in previous LCA studies (Quinn and Davis, 2015). The co-location of algal bioreactors with industrial waste CO2 could significantly reduce costs and environmental impacts. Pate et al. (2011) reported that for a lipid yield estimate of 19 m3 ha-1 year-1, results show that approximately 20 billion gallons of fuel can be produced annually without night storage, based on point-source CO2 availability constraints. Considering both land use and CO2 availability, we proposed that the massive algal production field could be located not in the advanced area like Southern China but instead in the North-Western region (Liang et al., 2013; Guo, 2019).
Autotrophic vs. heterotrophic styles
Some microalgae could grow under autotrophic and heterotrophic conditions (Smetana et al., 2017; Lu et al., 2021; Dai et al., 2022). A new functional food, Euglena gracilis, shows significantly different cellular components under autotrophic and heterotrophic cultivation, such as high contents of proteins (~55% DCW) in green Euglena powder (autotrophic) and 75%+ β-1,3-glucan contents in yellow powder (heterotrophic) (Dai et al., 2022). In this case, different product requirements from E. gracilis, proteins, or glucan determine the cultivation style. Heterotrophic cultivation also has its own advantages, especially for total fatty acid and lipid production. For some heterotrophic microalgae, such as Auxenochlorella protothecoides, the lipid content could reach up to 60% and total lipid productivity far higher than that of autotrophic algae (Xiong et al., 2008). Furthermore, algal dark fermentation generates higher neutral lipids and triglycerides and lower phospholipids and proteins than autotrophic algae (Wu et al., 2015). Otherwise, the environmental compacts under different cultivation styles should be carefully evaluated. A previous study indicated high impacts related to heat and energy use (autotrophic) and glucose for microalgal feed (heterotrophic) (Smetana et al., 2017). On the other hand, oil produced from heterotrophic Chlorella sp. only had lower impacts in acidification, eutrophication, carcinogens, and ecotoxicity when compared to the autotrophic Chlorella sp. (Lu et al., 2021). Due to its high biomass density, the harvest stage with a drying step for heterotrophic algae certainly has fewer impacts than autotrophic algae (Aggarwal et al., 2021; Lu et al., 2021). Together with a much lower water content of fermentative algae than autotrophic algae, a better economic and energy performance than autotrophic algae for bio-oil production can be concluded, although carbon source (e.g., glucose) is needed for heterotrophic algal growth and lipid biosynthesis (Lu et al., 2021).
Conclusions
This study focused on the comparative LCA of autotrophic cultivated microalgae coupled with CO2 capture and various downstream processes for algal slurry, algal powder, total fatty acid, lipids, bio-oil, and tablet production. This is also the limitation of this study with separated data collected from different countries and regions. With more consistent system boundaries, the same production pathway architecture, standards, and bigger databases, LCA of microalgae could be more helpful and useful for a greener earth planet.
Based on these comparisons and analyses, we proposed several recommendations for massive autotrophic cultivation improvement: i) strain development, optimum algal cultivation, biomass separation, and drying techniques with low environmental impacts; ii) use mainly of WW for algal cultivation; iii) careful final product selection which could lower the microalgal industry environmental impacts, making them more environmentally friendly, in which bioplastics could be one option if LCA verifies; iv) land selection plus electricity renewable energy such as photovoltaic systems, together with thermal power plants nearby for CO2 or flue gas.
Afterward, the autotrophic algal cultivation system would considerably achieve an energy-efficient, sustainable, and negative CO2 production green production process.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DZ and JW, conceptualization, investigation, methodology, formal analysis, data curation, writing—review and editing. SA, conceptualization, investigation, methodology, data curation, software, visualization, writing—review and editing. RY, WF, YH, conceptualization, investigation, methodology, formal analysis, data curation, writing—review and editing. ZC, writing—review and editing. MD, data curation, visualization. AL, conceptualization, formal analysis, writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Natural Science Basic Research Program of Shaanxi (Program No. 2022JQ-571), China’s National Key R&D Programs (2018YFA0902500, 2020YFA0908703, 2021YFA0910800), and the National Natural Science Foundation of China (41876188).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.990635/full#supplementary-material
References
Adesanya V. O., Cadena E., Scott S. A., Smith A. G. (2014). Life cycle assessment on microalgal biodiesel production using a hybrid cultivation system. Bioresour Technol. 163, 343–355. doi: 10.1016/j.biortech.2014.04.051
Aggarwal C., Singh D., Soni H., Pal A. (2021). “Heterotrophic cultivation of microalgae in wastewater,” in Recent advances in mechanical engineering. Eds. Kumar A., Pal A., Kachhwaha S. S., Jain P. K. (Springer Nature Singapore), 493–506.
Amorim M. L., Soares J., Coimbra J., Leite M. O., Albino L. F. T., Martins M. A. (2021). Microalgae proteins: production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 61 (12), 1976–2002. doi: 10.1080/10408398.2020.1768046
Asadi P., Rad H. A., Qaderi F. (2019). Comparison of chlorella vulgaris and chlorella sorokiniana pa.91 in post treatment of dairy wastewater treatment plant effluents. Environ. Sci. pollut. Res. Int. 26 (28), 29473–29489. doi: 10.1007/s11356-019-06051-8
Beckstrom B. D., Wilson M. H., Crocker M., Quinn J. C. (2020). Bioplastic feedstock production from microalgae with fuel co-products: A techno-economic and life cycle impact assessment. Algal Res. 46, 101769. doi: 10.1016/j.algal.2019.101769
Bhatt N. C., Panwar A., Bisht T. S., Tamta S. (2014). Coupling of algal biofuel production with wastewater. Sci. World J. 2014, 210504. doi: 10.1155/2014/210504
Bradley T., Maga D., Antón S. (2015). Unified approach to life cycle assessment between three unique algae biofuel facilities. Appl. Energy 154, 1052–10 61. doi: 10.1016/j.apenergy.2014.12.087
Bussa M., Zollfrank C., Röder H. (2021). Life cycle assessment with parameterised inventory to derive target values for process parameters of microalgae biorefineries. Algal Res. 57, 102352. doi: 10.1016/j.algal.2021.102352
Campbell P. K., Beer T., Batten D. (2011). Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour Technol. 102 (1), 50–56. doi: 10.1016/j.biortech.2010.06.048
Castro J. S., Calijuri M. L., Ferreira J., Assemany P. P., Ribeiro V. J. (2020). Microalgae based biofertilizer: A life cycle approach. Sci. Total Environ. 724, 138138. doi: 10.1016/j.scitotenv.2020.138138
Chen C. Y., Kuo E. W., Nagarajan D., Ho S. H., Dong C. D., Lee D. J., et al. (2020). Cultivating chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour Technol. 302, 122814. doi: 10.1016/j.biortech.2020.122814
Chen P. H., Quinn J. C. (2021). Microalgae to biofuels through hydrothermal liquefaction: Open-source techno-economic analysis and life cycle assessment. Appl. Energy 289, 116613. doi: 10.1016/j.apenergy.2021.116613
Chiaramonti D., Maniatis K., Tredici M. R., Verdelho V., Yan J. (2015). Life cycle assessment of algae biofuels: Needs and challenges. Appl. Energy 154, 1049–1051. doi: 10.1016/j.apenergy.2015.06.006
Chia S. R., Nomanbhay S., Chew K. W., Munawaroh H. S.H., Shamsuddin A. H., Show P. L. (2022). Algae as potential feedstock for various bioenergy production. Chemosphere 287(Pt 1), 131944. doi: 10.1016/j.chemosphere.2021.131944
Chong J. W.R., Khoo K. S., Yew G. Y., Leong W. H., Lim J. W., Lam M. K., et al. (2021a). Advances in production of bioplastics by microalgae using food waste hydrolysate and wastewater: A review. Bioresour Technol 342, 125947. doi: 10.1016/j.biortech.2021.125947.
Chong J. W.R., Yew G. Y., Khoo K. S., Ho S. H., Show P. L. (2021b). Recent advances on food waste pretreatment technology via microalgae for source of polyhydroxyalkanoates. J Environ Manage 293, 112782. doi: 10.1016/j.jenvman.2021.112782
Chong J. W.R., Tan X., Khoo K. S., Ng H. S., Jonglertjunya W., Yew G. Y., et al. (2022). Microalgae-based bioplastics: Future solution towards mitigation of plastic wastes. Environ Res 206, 112620. doi: 10.1016/j.envres.2021.112620
Clarens A., Colosi L. (2013). Life Cycle Assessment of Algae-to-Energy Systems. In: Lee J. (eds) Advanced Biofuels and Bioproducts. (New York, NY: Springer). doi: 10.1007/978-1-4614-3348-4_32
Clarens A. F., Nassau H., Resurreccion E. P., White M. A., Colosi L. M. (2011). Environmental impacts of algae-derived biodiesel and bioelectricity for transportation. Environ. Sci. Technol. 45 (17), 7554–7560. doi: 10.1021/es200760n
Collet P., Helias A., Lardon L., Ras M., Goy R. A., Steyer J. P. (2011). Life-cycle assessment of microalgae culture coupled to biogas production. Bioresour Technol. 102 (1), 207–214. doi: 10.1016/j.biortech.2010.06.154
Collotta M., Champagne P., Mabee W., Tomasoni G., Leite G. B., Busi L., et al. (2017). Comparative LCA of flocculation for the harvesting of microalgae for biofuels production. Proc. CIRP 61, 756–760. doi: 10.1016/j.procir.2016.11.146
Dai J., He J., Chen Z., Qin H., Du M., Lei A., et al. (2022). Euglena gracilis promotes lactobacillus growth and antioxidants accumulation as a potential next generation prebiotic. Front. Nutr. Nutr. Microbes. 9, 864565. doi: 10.3389/fnut.2022.864565
Davis D., Morão A., Johnson J. K., Shen L. (2021). Life cycle assessment of heterotrophic algae omega-3. Algal Res. 60, 102494. doi: 10.1016/j.algal.2021.102494
Depra M. C., Dias R. R., Severo I. A., de Menezes C. R., Zepka L. Q., Jacob-Lopes E. (2020). Carbon dioxide capture and use in photobioreactors: The role of the carbon dioxide loads in the carbon footprint. Bioresour Technol. 314, 123745. doi: 10.1016/j.biortech.2020.123745
Ding J., Hu X., Feng Z., Dong L. (2022). Environmental life cycle assessment of monosodium glutamate production in China: Based on the progress of cleaner production in recent ten years. Sci. Total Environ. 818, 151706. doi: 10.1016/j.scitotenv.2021.151706
Dolganyuk V., Belova D., Babich O., Prosekov A., Ivanova S., Katserov D., et al. (2020). Microalgae: A promising source of valuable bioproducts. Biomolecules 10, (8). doi: 10.3390/biom10081153
Dutta S., Neto F., Coelho M. C. (2016). Microalgae biofuels: A comparative study on techno-economic analysis & life-cycle assessment. Algal Res. 20, 44–52. doi: 10.1016/j.algal.2016.09.018
Fortier M.-O. P., Roberts G. W., Stagg-Williams S. M., Sturm B. S. M. (2014). Life cycle assessment of bio-jet fuel from hydrothermal liquefaction of microalgae. Appl. Energy 122, 73–82. doi: 10.1016/j.apenergy.2014.01.077
Foteinis S., Antoniadis-Gavriil A., Tsoutsos T. (2018). Life cycle assessment of algae-to-biodiesel shallow pond production systems in the Mediterranean: influence of species, pond type, by(co)-product valorisation and electricity mix. Biofuels Bioproducts Biorefining 12 (4), 542–558. doi: 10.1002/bbb.1871
Gaber K., Rösch C., Biondi N. (2021). Life cycle assessment of total fatty acid (TFA) production from microalgae Nannochloropsis oceanica at different sites and under different sustainability scenarios. Bioenergy Res. doi: 10.1007/s12155-021-10279-z
Gnansounou E., Kenthorai Raman J. (2016). Life cycle assessment of algae biodiesel and its co-products. Appl. Energy 161, 300–308. doi: 10.1016/j.apenergy.2015.10.043
Grierson S., Strezov V., Bengtsson J. (2013). Life cycle assessment of a microalgae biomass cultivation, bio-oil extraction and pyrolysis processing regime. Algal Res. 2 (3), 299–311. doi: 10.1016/j.algal.2013.04.004
Guiton M., Suárez-Montes D., Sánchez R., Baustert P., Soukoulis C., Okan B. S., et al. (2022). Comparative life cycle assessment of a microalgae-based oil metal working fluid with its petroleum-based and vegetable-based counterparts. J. Cleaner Production 338, 130506. doi: 10.1016/j.jclepro.2022.130506
Guo L. (2019). Study on environmental impact of carbon fixation and energy utilization by microalgae based on LCA method (Harbin Industry University).
Guo S., Li X., Zhao R., Gong Y. (2021). Comparison of life cycle assessment between lyocell fiber and viscose fiber in China. Int. J. Life Cycle Assess. 26 (8), 1545–1555. doi: 10.1007/s11367-021-01916-y
Guo F., Zhao J A. L., Yang X. (2016). Life cycle assessment of microalgae-based aviation fuel: Influence of lipid content with specific productivity and nitrogen nutrient effects. Bioresour Technol. 221, 350–357. doi: 10.1016/j.biortech.2016.09.044
Hosseinzadeh-Bandbafha H., Tabatabaei M., Aghbashlo M., Sulaiman A., Ghassemi A. (2020). Life-cycle assessment (LCA) analysis of algal fuels. Methods Mol. Biol. 1980, 121–151. doi: 10.1007/7651_2018_204
IKE (2012a) Chinese Life cycle database–CLCD. Available at: http://www.ike-global.com/products-2/chinese-lcadatabase-clcd (Accessed March 2015).
IKE (2012b) Introduction of life cycle energy conservation & emission Reduction(ECER) assessment (in Chinese). Available at: http://goo.gl/pNX1Bs (Accessed October 2014).
Jian H., Jing Y., Peidong Z. (2015). Life cycle analysis on fossil energy ratio of algal biodiesel: effects of nitrogen deficiency and oil extraction technology. Sci. World J. 2015, 920968. doi: 10.1155/2015/920968
Jiao J., Li J., Bai Y. (2019). Uncertainty analysis in the life cycle assessment of cassava ethanol in China. J. Cleaner Production 206, 438–451. doi: 10.1016/j.jclepro.2018.09.199
Khan M. I., Shin J. H., Kim J. D. (2018). The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact 17 (1), 36. doi: 10.1186/s12934-018-0879-x
Khoo K.S., Ooi C.W., Chew K.W., Foo S.C., Show P.L. (2021). Bioprocessing of Chaetoceros calcitrans for the recovery of fucoxanthin using CO(2)-based alkyl carbamate ionic liquids. Bioresour Technol 322, 124520. doi: 10.1016/j.biortech.2020.124520
Kumar V., Karela R. P., Korstad J., Kumar S., Srivastava R., Bauddh K. (2017). Ecological, Economical and Life Cycle Assessment of Algae and Its Biofuel. In: Gupta S., Malik A., Bux F. (eds) Algal Biofuels. Springer, Cham. doi: 10.1007/978-3-319-51010-1_21
Liang S., Xu M., Zhang T. (2013). Life cycle assessment of biodiesel production in China. Bioresour Technol. 129, 72–77. doi: 10.1016/j.biortech.2012.11.037
Lu Y., Mu D., Xue Z., Xu P., Li Y., Xiang W., et al. (2021). Life cycle assessment of industrial production of microalgal oil from heterotrophic fermentation. Algal Res. 58, 102404. doi: 10.1016/j.algal.2021.102404
Matos ÂP (2019). “Chapter 3 - microalgae as a potential source of proteins,” in Proteins: Sustainable source, processing and applications. Ed. Galanakis C. M. (Academic Press), pp 63–pp 96. doi: 10.1016/B978-0-12-816695-6.00003-9
Mediboyina M. K., Banuvalli B. K., Chauhan V. S., Mudliar S. N. (2020). Comparative life cycle assessment of autotrophic cultivation of Scenedesmus dimorphus in raceway pond coupled to biodiesel and biogas production. Bioprocess Biosyst. Eng. 43 (2), 233–247. doi: 10.1007/s00449-019-02220-8
Mi Z., Kunliang L. (2019). “Feng p whole life cycle comprehensive environment impact assessment of hydraulic power chuck,” in 2019 2nd Asia Conference on Energy and Environment Engineering (ACEEE), Vol. 2019. 35–39. (Hiroshima, Japan: IEEE). doi: 10.1109/ACEEE.2019.8816876
Mohseni A., Fan L., Roddick F. A. (2021). Impact of microalgae species and solution salinity on algal treatment of wastewater reverse osmosis concentrate. Chemosphere 285, 131487. doi: 10.1016/j.chemosphere.2021.131487
Moody J. W., McGinty C. M., Quinn J. C. (2014). Global evaluation of biofuel potential from microalgae. Proc. Natl. Acad. Sci. U.S.A. 111 (23), 8691–8696. doi: 10.1073/pnas.1321652111
Nawkarkar P., Singh A. K., Abdin M. Z., Kumar S. (2019). Life cycle assessment of Chlorella species producing biodiesel and remediating wastewater. J. Biosci. 44, (4). doi: 10.1007/s12038-019-9896-0
Onorato C., Rösch C. (2020). Comparative life cycle assessment of astaxanthin production with Haematococcus pluvialis in different photobioreactor technologies. Algal Res. 50, 102005. doi: 10.1016/j.algal.2020.102005
Pate R., Klise G., Wu B. (2011). Resource demand implications for US algae biofuels production scale-up. Appl. Energy 88 (10), 3377–3388. doi: 10.1016/j.apenergy.2011.04.023
Patidar S. K., Mishra S. (2017). “Carbon sequestration by microalgae: A green approach for climate change mitigation,” in Encyclopedia of sustainable technologies. Ed. Abraham M. A. (Oxpford: Elsevier), pp 477–pp 483. Available at: 10.1016/B978-0-12-409548-9.10125-3.
Pérez-López P., González-García S., Jeffryes C., Agathos S. N., McHugh E., Walsh D., et al. (2014). Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: from lab to pilot scale. J. Cleaner Production 64, 332–344. doi: 10.1016/j.jclepro.2013.07.011
Pires J. C. M. (2017). COP21: The algae opportunity? Renewable Sustain. Energy Rev. 79, 867–877. doi: 10.1016/j.rser.2017.05.197
Podder M. S., Majumder C. B. (2016). The use of artificial neural network for modelling of phycoremediation of toxic elements As(III) and As(V) from wastewater using botryococcus braunii. Spectrochim Acta A Mol. Biomol Spectrosc 155, 130–145. doi: 10.1016/j.saa.2015.11.011
Porcelli R., Dotto F., Pezzolesi L., Marazza D., Greggio N., Righi S. (2020). Comparative life cycle assessment of microalgae cultivation for non-energy purposes using different carbon dioxide sources. Sci. Total Environ. 721, 137714. doi: 10.1016/j.scitotenv.2020.137714
Quinn J. C., Catton K. B., Wagner N. A., Bradley T. H. (2011). Current Large-scale US biofuel potential from microalgae cultivated in photobioreactors. Bioenergy Res. 5, 49–60. doi: 10.1007/s12155-011-9165-z
Quinn J. C., Davis R. (2015). The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling. Bioresour Technol. 184, 444–452. doi: 10.1016/j.biortech.2014.10.075
Sandmann M., Smetana S., Heinz V., Rohn S. (2021). Comparative life cycle assessment of a mesh ultra-thin layer photobioreactor and a tubular glass photobioreactor for the production of bioactive algae extracts. Bioresour Technol. 340, 125657. doi: 10.1016/j.biortech.2021.125657
Schade S., Meier T. (2020). Distinct microalgae species for food–part 1: a methodological (top-down) approach for the life cycle assessment of microalgae cultivation in tubular photobioreactors. J. Appl. Phycol 32 (5), 2977–2995. doi: 10.1007/s10811-020-02177-2
Schade S., Stangl G. I., Meier T. (2020). Distinct microalgae species for food–part 2: comparative life cycle assessment of microalgae and fish for eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and protein. J. Appl. Phycol 32 (5), 2997–3013. doi: 10.1007/s10811-020-02181-6
Schneider R., de Moura Lima M., Hoeltz M., de Farias Neves F., John D. K., de Azevedo A. (2018). Life cycle assessment of microalgae production in a raceway pond with alternative culture media. Algal Res. 32, 280–292. doi: 10.1016/j.algal.2018.04.012
Shi R., Handler R. M., Shonnard D. R. (2019). Life cycle assessment of novel technologies for algae harvesting and oil extraction in the renewable diesel pathway. Algal Res. 37, 248–259. doi: 10.1016/j.algal.2018.12.005
Smetana S., Sandmann M., Rohn S., Pleissner D., Heinz V. (2017). Autotrophic and heterotrophic microalgae and cyanobacteria cultivation for food and feed: life cycle assessment. Bioresource Technol. 245, 162–170. doi: 10.1016/j.biortech.2017.08.113
Somers M. D., Chen P., Clippinger J., Cruce J. R., Davis R., Lammers P. J., et al. (2021). Techno-economic and life-cycle assessment of fuel production from mixotrophic Galdieria sulphuraria microalgae on hydrolysate. Algal Res. 59, 102419. doi: 10.1016/j.algal.2021.102419
Wu C., Xiong W., Dai J., Wu Q. (2015). Genome-based metabolic mapping and 13C flux analysis reveal systematic properties of an oleaginous microalga chlorella protothecoides. Plant Physiol. 167 (2), 586–599. doi: 10.1104/pp.114.250688
Xiong W., Li X., Xiang J., Wu Q. (2008). High-density fermentation of microalga chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol. 78 (1), 29–36. doi: 10.1007/s00253-007-1285-1
Ye S., Gao L., Zhao J., An M., Wu H., Li M. (2020). Simultaneous wastewater treatment and lipid production by scenedesmus sp. HXY2. Bioresour Technol. 302, 122903. doi: 10.1016/j.biortech.2020.122903
Ye C., Mu D., Horowitz N., Xue Z., Chen J., Xue M., et al. (2018). Life cycle assessment of industrial scale production of spirulina tablets. Algal Res. 34, 154–163. doi: 10.1016/j.algal.2018.07.013
Keywords: microalgae cultivation, life cycle assessment, biofuels, carbon dioxide mitigation, biomass, bioactive compounds
Citation: Zhang D, An S, Yao R, Fu W, Han Y, Du M, Chen Z, Lei A and Wang J (2022) Life cycle assessment of auto-tropically cultivated economic microalgae for final products such as food, total fatty acids, and bio-oil. Front. Mar. Sci. 9:990635. doi: 10.3389/fmars.2022.990635
Received: 10 July 2022; Accepted: 04 August 2022;
Published: 25 August 2022.
Edited by:
Weiqi Fu, Zhejiang University, ChinaReviewed by:
Pau Loke Show, University of Nottingham Malaysia Campus, MalaysiaQuanyu Zhao, Nanjing Tech University, China
Copyright © 2022 Zhang, An, Yao, Fu, Han, Du, Chen, Lei and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangxin Wang, jwang88@tju.edu.cn
 Dan Zhang1
Dan Zhang1  Zixi Chen
Zixi Chen Anping Lei
Anping Lei Jiangxin Wang
Jiangxin Wang