Human Health and Soil Health Risks from Heavy Metals, Micro(nano)plastics, and Antibiotic Resistant Bacteria in Agricultural Soils
Abstract
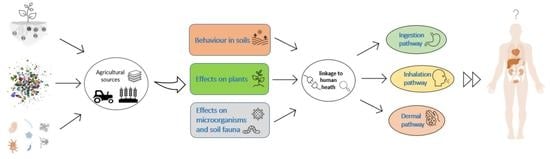
:1. Introduction
- (1)
- Investigate agriculturally related sources of contamination with HMs, MNPs, and ARB.
- (2)
- Analyse their effects on human health differentiated by uptake pathway (i.e., oral, respiratory, dermal), as well as interactions with soil microorganisms, fauna, and plants.
- (3)
- Identify current knowledge gaps relevant for assessing the linkages between soil contamination and human health.
2. Material and Methods
3. Results and Discussion
3.1. Heavy Metals
3.1.1. Sources of HMs in Agricultural Soils
3.1.2. Linkages between HMs and Human Health
3.1.3. Behaviour of HMs in Soils
3.1.4. HM Effects on Flora and Fauna
Effects on Plants
Effects on Microorganisms and Soil Fauna
3.2. Micro(nano)plastics
3.2.1. Sources of MNPs in Agricultural Soils
3.2.2. Linkages between MNPs and Human Health
3.2.3. Behaviour of MNPs in Soils
3.2.4. MNP Effects on Flora and Fauna
Effects on Plants
Effects on Microorganisms and Soil Fauna
3.3. Antibiotic-Resistant Bacteria
3.3.1. Sources of ARB in Agricultural Soils
3.3.2. Linkages between ARB and Human Health
3.3.3. Behaviour of ARB in Soils
3.3.4. ARB Effects on Flora and Fauna
Effects on Plants
Effects on Microorganisms and Soil Fauna
4. Synthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.-G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef] [PubMed]
- Karimyan, K.; Alimohammadi, M.; Maleki, A.; Yunesian, M.; Nodehi, R.N.; Foroushani, A.R. Human health and ecological risk assessment of heavy metal(loid)s in agricultural soils of rural areas: A case study in Kurdistan Province, Iran. J. Environ. Health Sci. Eng. 2020, 18, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2017, 69, 159–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brevik, E.C.; Sauer, T.J. The past, present, and future of soils and human health studies. Soil 2015, 1, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and Human Health: Current Status and Future Needs. Air Soil Water Res. 2020, 13, 4441. [Google Scholar] [CrossRef]
- Sanaei, F.; Amin, M.M.; Alavijeh, Z.P.; Esfahani, R.A.; Sadeghi, M.; Bandarrig, N.S.; Fatehizadeh, A.; Taheri, E.; Rezakazemi, M. Health risk assessment of potentially toxic elements intake via food crops consumption: Monte Carlo simulation-based probabilistic and heavy metal pollution index. Environ. Sci. Pollut. Res. 2020, 28, 1479–1490. [Google Scholar] [CrossRef]
- Duffus, J.H. Heavy metals—A meaningless term? (IUPAC technical report). Pure Appl. Chem. 2003, 75, 1357. [Google Scholar] [CrossRef]
- Alloway, B.J. Introduction; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–9. [Google Scholar]
- Balseiro-Romero, M.; Baveye, P.C. Book Review: Soil Pollution: A Hidden Danger Beneath our Feet. Front. Environ. Sci. 2018, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Demková, L.; Árvay, J.; Bobuľská, L.; Tomáš, J.; Stanovič, R.; Lošák, T.; Harangozo, L.; Vollmannová, A.; Bystrická, J.; Musilová, J.; et al. Accumulation and environmental risk assessment of heavy metals in soil and plants of four different ecosystems in a former polymetallic ores mining and smelting area (Slovakia). J. Environ. Sci. Health Part A 2017, 52, 479–490. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2020, 267, 129205. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Ma, J.; Wu, X.; Ju, T.; Lin, X.; Zhang, Y.; Li, X.; Gong, Y.; Hou, H.; Zhao, L.; et al. Inventories of heavy metal inputs and outputs to and from agricultural soils: A review. Ecotoxicol. Environ. Saf. 2018, 164, 118–124. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef] [Green Version]
- Alengebawy, A.; Abdelkhalek, S.; Qureshi, S.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Xiao, P.; Zhou, Y.; Li, X.; Xu, J.; Zhao, C. Assessment of Heavy Metals in Agricultural Land: A Literature Review Based on Bibliometric Analysis. Sustainability 2021, 13, 4559. [Google Scholar] [CrossRef]
- Bartkova, S.; Kahru, A.; Heinlaan, M.; Scheler, O. Techniques Used for Analyzing Microplastics, Antimicrobial Resistance and Microbial Community Composition: A Mini-Review. Front. Microbiol. 2021, 12, 603967. [Google Scholar] [CrossRef]
- Ullah, R.; Tsui, M.T.; Chen, H.; Chow, A.; Williams, C.; Ligaba-Osena, A. Microplastics interaction with terrestrial plants and their impacts on agriculture. J. Environ. Qual. 2021, 50, 1024–1041. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Pathan, S.; Arfaioli, P.; Bardelli, T.; Ceccherini, M.; Nannipieri, P.; Pietramellara, G. Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard. Sustainability 2020, 12, 7255. [Google Scholar] [CrossRef]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total. Environ. 2021, 805, 150431. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allouzi, M.M.A.; Tang, D.Y.Y.; Chew, K.W.; Rinklebe, J.; Bolan, N.; Allouzi, S.M.A.; Show, P.L. Micro (nano) plastic pollution: The ecological influence on soil-plant system and human health. Sci. Total Environ. 2021, 788, 147815. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Etienne-Mesmin, L.; Grootaert, C.; Jelsbak, L.; Syberg, K.; Blanquet-Diot, S.; Mercier-Bonin, M. Microplastics in the human digestive environment: A focus on the potential and challenges facing in vitro gut model development. J. Hazard. Mater. 2021, 415, 125632. [Google Scholar] [CrossRef]
- Yee, M.; Hii, L.-W.; Looi, C.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Maraveas, C. The Sustainability of Plastic Nets in Agriculture. Sustainability 2020, 12, 3625. [Google Scholar] [CrossRef]
- Silva, G.; Madrid, F.G.; Hernández, D.; Pincheira, G.; Peralta, A.; Gavilán, M.U.; Vergara-Carmona, V.; Fuentes-Peñailillo, F. Microplastics and Their Effect in Horticultural Crops: Food Safety and Plant Stress. Agronomy 2021, 11, 1528. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Kumari, A.; Rajput, V.D.; Mandzhieva, S.S.; Rajput, S.; Minkina, T.; Kaur, R.; Sushkova, S.; Kumari, P.; Ranjan, A.; Kalinitchenko, V.P.; et al. Microplastic Pollution: An Emerging Threat to Terrestrial Plants and Insights into Its Remediation Strategies. Plants 2022, 11, 340. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; Álvarez-Méndez, S.J.; Kropp, R.M.; Perdomo-González, A.; Hernández-Borges, J.; Díaz-Peña, F.J. Microplastics in Agricultural Systems: Analytical Methodologies and Effects on Soil Quality and Crop Yield. Agriculture 2022, 12, 1162. [Google Scholar] [CrossRef]
- Gudda, F.O.; Waigi, M.G.; Odinga, E.S.; Yang, B.; Carter, L.; Gao, Y. Antibiotic-contaminated wastewater irrigated vegetables pose resistance selection risks to the gut microbiome. Environ. Pollut. 2020, 264, 114752. [Google Scholar] [CrossRef]
- He, J.; Yan, Z.; Chen, Q. Transmission of antibiotic resistance genes in agroecosystems: An overview. Front. Agric. Sci. Eng. 2020, 7, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Jechalke, S.; Heuer, H.; Siemens, J.; Amelung, W.; Smalla, K. Fate and effects of veterinary antibiotics in soil. Trends Microbiol. 2014, 22, 536–545. [Google Scholar] [CrossRef]
- Ondon, B.S.; Li, S.; Zhou, Q.; Li, F. Sources of Antibiotic Resistant Bacteria (ARB) and Antibiotic Resistance Genes (ARGs) in the Soil: A Review of the Spreading Mechanism and Human Health Risks. Rev. Environ. Contam. Toxicol. Vol. 2021, 256, 121–153. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, M.-U.; Luies, S.K.; Kamal, A.; Ferdous, S.; Lin, A.; Sharior, F.; Khan, R.; Rahman, Z.; Parvez, S.M.; et al. Contamination of Fresh Produce with Antibiotic-Resistant Bacteria and Associated Risks to Human Health: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 19, 360. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; Araujo, A.; Singh, R.P. Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Abreu-Junior, C.H.; Brossi, M.J.D.L.; Monteiro, R.T.; Cardoso, P.H.S.; Mandu, T.D.S.; Nogueira, T.A.R.; Ganga, A.; Filzmoser, P.; de Oliveira, F.C.; Firme, L.P.; et al. Effects of sewage sludge application on unfertile tropical soils evaluated by multiple approaches: A field experiment in a commercial Eucalyptus plantation. Sci. Total Environ. 2018, 655, 1457–1467. [Google Scholar] [CrossRef]
- Giannakis, I.; Emmanouil, C.; Mitrakas, M.; Manakou, V.; Kungolos, A. Chemical and ecotoxicological assessment of sludge-based biosolids used for corn field fertilization. Environ. Sci. Pollut. Res. 2020, 28, 3797–3809. [Google Scholar] [CrossRef]
- Niño-Savala, A.G.; Zhuang, Z.; Ma, X.; Fangmeier, A.; Li, H.; Tang, A.; Liu, X. Cadmium pollution from phosphate fertilizers in arable soils and crops: An overview. Front. Agric. Sci. Eng. 2019, 6, 419. [Google Scholar] [CrossRef] [Green Version]
- Marini, M.; Caro, D.; Thomsen, M. The new fertilizer regulation: A starting point for cadmium control in European arable soils? Sci. Total Environ. 2020, 745, 140876. [Google Scholar] [CrossRef] [PubMed]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Jastrzębska, M.; Kostrzewska, M.K.; Saeid, A.; Jastrzębski, W.P. Do New-Generation Recycled Phosphorus Fertilizers Increase the Content of Potentially Toxic Elements in Soil and Plants? Minerals 2021, 11, 999. [Google Scholar] [CrossRef]
- Gianico, A.; Braguglia, C.; Gallipoli, A.; Montecchio, D.; Mininni, G. Land Application of Biosolids in Europe: Possibilities, Con-Straints and Future Perspectives. Water 2021, 13, 103. [Google Scholar] [CrossRef]
- Hušek, M.; Moško, J.; Pohořelý, M. Sewage sludge treatment methods and P-recovery possibilities: Current state-of-the-art. J. Environ. Manag. 2022, 315, 115090. [Google Scholar] [CrossRef]
- Provolo, G.; Manuli, G.; Finzi, A.; Lucchini, G.; Riva, E.; Sacchi, G.A. Effect of Pig and Cattle Slurry Application on Heavy Metal Composition of Maize Grown on Different Soils. Sustainability 2018, 10, 2684. [Google Scholar] [CrossRef] [Green Version]
- Nag, R.; Cummins, E. Human health risk assessment of lead (Pb) through the environmental-food pathway. Sci. Total Environ. 2021, 810, 151168. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2019, 30, 167–176. [Google Scholar] [CrossRef]
- Hassanien, M.A. Atmospheric Heavy Metals Pollution: Exposure and Prevention Policies in Mediterranean Basin. In Environmental Heavy Metal Pollution and Effects on Child Mental Development; Springer: Dordrecht, The Netherlands, 2010; Volume 1, pp. 287–307. [Google Scholar]
- Doabi, S.A.; Karami, M.; Afyuni, M.; Yeganeh, M. Pollution and health risk assessment of heavy metals in agricultural soil, atmospheric dust and major food crops in Kermanshah province, Iran. Ecotoxicol. Environ. Saf. 2018, 163, 153–164. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, G.N.; Ahmed, M.B.M.; Marrez, D.A. Reduction of heavy metals content in contaminated vegetables due to the post-harvest treatments. Egypt. J. Chem. 2018, 61, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- JSmith, J.P.; Brabander, D.J.; Panek, L.A.; Besancon, J.R. Enrichment of potentially toxic elements in the fine fraction of soils from Iraq and Kuwait. J. Soils Sediments 2019, 19, 3545–3563. [Google Scholar] [CrossRef]
- Sah, D.; Verma, P.K.; Kandikonda, M.K.; Lakhani, A. Pollution characteristics, human health risk through multiple exposure pathways, and source apportionment of heavy metals in PM10 at Indo-Gangetic site. Urban Clim. 2018, 27, 149–162. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Alattar, M.; Jiang, S.; Han, J.; Ma, Y.; Jiang, C. The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to PM2.5 in rats. Sci. Rep. 2015, 5, 16936. [Google Scholar] [CrossRef] [Green Version]
- Ghazali, A.R.; Razak, N.E.A.; Othman, M.S.; Othman, H.; Ishak, I.; Lubis, S.H.; Mohammad, N.; Hamid, Z.A.; Harun, Z.; Kamarulzaman, F.; et al. Study of Heavy Metal Levels among Farmers of Muda Agricultural Development Authority, Malaysia. J. Environ. Public Health 2012, 2012, 758349. [Google Scholar] [CrossRef] [Green Version]
- Feszterová, M.; Porubcová, L.; Tirpáková, A. The Monitoring of Selected Heavy Metals Content and Bioavailability in the Soil-Plant System and Its Impact on Sustainability in Agribusiness Food Chains. Sustainability 2021, 13, 7021. [Google Scholar] [CrossRef]
- Abdel-Rahman, G.N.-E. Heavy metals, definition, sources of food contamination, incidence, impacts and remediation: A literature review with recent updates. Egypt. J. Chem. 2021, 65, 1419–1437. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Gruszecka-Kosowska, A. Potentially Harmful Element Concentrations in the Vegetables Cultivated on Arable Soils, with Human Health-Risk Implications. Int. J. Environ. Res. Public Health 2019, 16, 4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beglaryan, M.; Pipoyan, D.; Tepanosyan, G.; Sahakyan, L. Toxic element contents and associated multi-medium health risk assessment in an area under continuous agricultural use. Environ. Monit. Assess. 2022, 194, 184. [Google Scholar] [CrossRef] [PubMed]
- Puschenreiter, M.; Horak, O.; Friesl, W.; Hartl, W. Low-cost agricultural measures to reduce heavy metal transfer into the food chain—A review. Plant Soil Env. 2005, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bao, J.; Wang, T.; Moryani, H.; Kang, W.; Zheng, J.; Zhan, C.; Xiao, W. Hazardous Heavy Metals Accumulation and Health Risk Assessment of Different Vegetable Species in Contaminated Soils from a Typical Mining City, Central China. Int. J. Environ. Res. Public Health 2021, 18, 2617. [Google Scholar] [CrossRef]
- Franzaring, J.; Fangmeier, A.; Schlosser, S.; Hahn, V. Cadmium concentrations in German soybeans are elevated in conurbations and in regions dominated by mining and the metal industry. J. Sci. Food Agric. 2018, 99, 3711–3715. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Lv, Y.; Liu, X.; Zhong, J.; Cui, X.; Zhang, M.; Ma, D.; Yan, X.; Zhu, X. Effects of Heavy Metals/Metalloids and Soil Properties on Microbial Communities in Farmland in the Vicinity of a Metals Smelter. Front. Microbiol. 2021, 12, 707786. [Google Scholar] [CrossRef]
- Hodson, M.E. Effects of Heavy Metals and Metalloids on Soil Organisms; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–160. [Google Scholar]
- Henao, S.G.; Ghneim-Herrera, T. Heavy Metals in Soils and the Remediation Potential of Bacteria Associated with the Plant Microbiome. Front. Environ. Sci. 2021, 9, 604216. [Google Scholar] [CrossRef]
- Nahmani, J.; Lavelle, P. Effects of heavy metal pollution on soil macrofauna in a grassland of Northern France. Eur. J. Soil Biol. 2002, 38, 297–300. [Google Scholar] [CrossRef]
- Okeke, E.S.; Okoye, C.O.; Atakpa, E.O.; Ita, R.E.; Nyaruaba, R.; Mgbechidinma, C.L.; Akan, O.D. Microplastics in agroecosystems-impacts on ecosystem functions and food chain. Resour. Conserv. Recycl. 2021, 177, 105961. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total. Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Ng, E.-L.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total. Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, J.-S.; Lee, H.; Lee, H.-J. Abundance and characteristics of microplastics in soils with different agricultural practices: Importance of sources with internal origin and environmental fate. J. Hazard. Mater. 2020, 403, 123997. [Google Scholar] [CrossRef]
- Sarker, A.; Deepo, D.M.; Nandi, R.; Rana, J.; Islam, S.; Rahman, S.; Hossain, M.N.; Islam, S.; Baroi, A.; Kim, J.-E. A review of microplastics pollution in the soil and terrestrial ecosystems: A global and Bangladesh perspective. Sci. Total Environ. 2020, 733, 139296. [Google Scholar] [CrossRef]
- Isari, E.; Papaioannou, D.; Kalavrouziotis, I.; Karapanagioti, H. Microplastics in Agricultural Soils: A Case Study in Cultivation of Watermelons and Canning Tomatoes. Water 2021, 13, 2168. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, W.; Xu, E.G.; Li, L.; Zhang, H.; Yang, Y. Uptake, translocation, and biological impacts of micro(nano)plastics in terrestrial plants: Progress and prospects. Environ. Res. 2021, 203, 111867. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babou, E.; Hiskakis, M.; Kyrikou, I. Analysis of long-term degradation behaviour of polyethylene mulching films with pro-oxidants under real cultivation and soil burial conditions. Environ. Sci. Pollut. Res. 2015, 22, 2584–2598. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.; Debruyn, J.M. Biodegradable Plastic Mulch Films: Impacts on Soil Microbial Communities and Ecosystem Functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, C.; Devetter, L.; Ghimire, S.; Hayes, D. Suitability of Biodegradable Plastic Mulches for Organic and Sustainable Agricultural Production Systems. HortScience 2017, 52, 10–15. [Google Scholar] [CrossRef]
- Hayes, D.G. Enhanced end-of-life performance for biodegradable plastic mulch films through improving standards and addressing research gaps. Curr. Opin. Chem. Eng. 2021, 33, 100695. [Google Scholar] [CrossRef]
- Iqbal, R.; Raza, M.A.S.; Valipour, M.; Saleem, M.F.; Zaheer, M.S.; Ahmad, S.; Toleikiene, M.; Haider, I.; Aslam, M.U.; Nazar, M.A. Potential agricultural and environmental benefits of mulches—A review. Bull. Natl. Res. Cent. 2020, 44, 1–16. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [Green Version]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [Green Version]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Tan, W.; Zhang, Z. Microplastics as an Emerging Environmental Pollutant in Agricultural Soils: Effects on Ecosystems and Human Health. Front. Environ. Sci. 2022, 10, 855292. [Google Scholar] [CrossRef]
- Conti, G.O.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Santos, P.S.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment–Sources, fates and effects. Sci. Total. Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total. Environ. 2019, 702, 134455. [Google Scholar] [CrossRef]
- Fackelmann, G.; Sommer, S. Microplastics and the gut microbiome: How chronically exposed species may suffer from gut dysbiosis. Mar. Pollut. Bull. 2019, 143, 193–203. [Google Scholar] [CrossRef]
- Teles, M.; Balasch, J.C.; Oliveira, M.; Sardans, J.; Peñuelas, J. Insights into nanoplastics effects on human health. Sci. Bull. 2020, 65, 1966–1969. [Google Scholar] [CrossRef]
- Blackburn, K.; Green, D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio 2021, 51, 518–530. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Domenech, J.; Marcos, R. Pathways of human exposure to microplastics, and estimation of the total burden. Curr. Opin. Food Sci. 2021, 39, 144–151. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Joos, L.; De Tender, C. Soil under stress: The importance of soil life and how it is influenced by (micro)plastic pollution. Comput. Struct. Biotechnol. J. 2022, 20, 1554–1566. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the soil environment: Occurrence, risks, interactions and fate–A review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2175–2222. [Google Scholar] [CrossRef]
- Rezaei, M.; Riksen, M.J.; Sirjani, E.; Sameni, A.; Geissen, V. Wind erosion as a driver for transport of light density microplastics. Sci. Total. Environ. 2019, 669, 273–281. [Google Scholar] [CrossRef] [PubMed]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Jinjin, C.; Ji, R.; Ma, Y.; Yu, X. Microplastics in agricultural soils: Sources, effects, and their fate. Curr. Opin. Environ. Sci. Health 2021, 25, 100311. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Machado, A.A.D.S.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total. Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard Mater. 2021, 402, 124034. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Thapa, B.; Yang, X.; Gertsen, H.; Salánki, T.; Geissen, V.; Garbeva, P. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: A potential for soil restoration. Sci. Total. Environ. 2018, 624, 753–757. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhu, L.; Wang, J.; Xing, B. Antibiotic resistance in agricultural soils: Source, fate, mechanism and attenuation strategy. Crit. Rev. Environ. Sci. Technol. 2020, 52, 847–889. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total. Environ. 2017, 599, 500–512. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. MicrobiologyOpen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zhu, Y.-G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef]
- Münch, S.; Papke, N.; Thiel, N.; Nübel, U.; Siller, P.; Roesler, U.; Biniasch, O.; Funk, R.; Amon, T. Effects of farmyard manure application on dust emissions from arable soils. Atmospheric Pollut. Res. 2020, 11, 1610–1624. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.-H.; Qiao, M.; Chen, Z.; Su, J.-Q.; Zhu, Y.-G. Antibiotic resistance genes in manure-amended soil and vegetables at harvest. J. Hazard. Mater. 2015, 299, 215–221. [Google Scholar] [CrossRef]
- Wang, F.; Fu, Y.-H.; Sheng, H.-J.; Topp, E.; Jiang, X.; Zhu, Y.-G.; Tiedje, J.M. Antibiotic resistance in the soil ecosystem: A One Health perspective. Curr. Opin. Environ. Sci. Health 2021, 20, 100230. [Google Scholar] [CrossRef]
- Kabelitz, T.; Ammon, C.; Funk, R.; Münch, S.; Biniasch, O.; Nübel, U.; Thiel, N.; Rösler, U.; Siller, P.; Amon, B.; et al. Functional relationship of particulate matter (PM) emissions, animal species, and moisture content during manure application. Environ. Int. 2020, 143, 105577. [Google Scholar] [CrossRef]
- Tadić, D.; Hernandez, M.J.B.; Cerqueira, F.; Matamoros, V.; Piña, B.; Bayona, J.M. Occurrence and human health risk assessment of antibiotics and their metabolites in vegetables grown in field-scale agricultural systems. J. Hazard. Mater. 2021, 401, 123424. [Google Scholar] [CrossRef]
- Marti, R.; Scott, A.; Tien, Y.-C.; Murray, R.; Sabourin, L.; Zhang, Y.; Topp, E. Impact of Manure Fertilization on the Abundance of Antibiotic-Resistant Bacteria and Frequency of Detection of Antibiotic Resistance Genes in Soil and on Vegetables at Harvest. Appl. Environ. Microbiol. 2013, 79, 5701–5709. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2018, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Xie, J.; He, T.; Wu, D.; Li, X. Airborne transmission as an integral environmental dimension of antimicrobial resistance through the “One Health” lens. Crit. Rev. Environ. Sci. Technol. 2021, 52, 4172–4193. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Wang, M.; Chen, H.; Yang, Y.; Chen, Q.-L.; Yao, M. Time-resolved spread of antibiotic resistance genes in highly polluted air. Environ. Int. 2019, 127, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sorinolu, A.J.; Tyagi, N.; Kumar, A.; Munir, M. Antibiotic resistance development and human health risks during wastewater reuse and biosolids application in agriculture. Chemosphere 2020, 265, 129032. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2011, 32, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Zalewska, M.; Błażejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure–Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Sazykin, I.S.; Sazykina, M.A. The influence of heavy metals, polyaromatic hydrocarbons, and polychlorinated biphenyls pollution on the development of antibiotic resistance in soils. Environ. Sci. Pollut. Res. 2018, 25, 9283–9292. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, L.; Chen, L.; Li, S.; Sun, L. Bioaccumulation of antibiotics in crops under long-term manure application: Occurrence, biomass response and human exposure. Chemosphere 2018, 219, 882–895. [Google Scholar] [CrossRef]
- Mathews, S.; Reinhold, D. Biosolid-borne tetracyclines and sulfonamides in plants. Environ. Sci. Pollut. Res. 2013, 20, 4327–4338. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Yang, Q.; Sun, L.; Yang, X.; Zhou, M.; Deng, R.; Bi, L. Plant Growth, Antibiotic Uptake, and Prevalence of Antibiotic Resistance in an Endophytic System of Pakchoi under Antibiotic Exposure. Int. J. Environ. Res. Public Health 2017, 14, 1336. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, X.; Li, Y.; Liu, Y.; Sun, Y.; Xia, S.; Zhao, J. Effects of coexistence of tetracycline, copper and microplastics on the fate of antibiotic resistance genes in manured soil. Sci. Total. Environ. 2021, 790, 148087. [Google Scholar] [CrossRef]



| Search String: Contaminant Term AND Human Health Term AND Agricultural Soils Term | ||
|---|---|---|
| Contaminant Term (OR) | Human Health Term (OR) | Agricultural Soils Term (OR) |
| “human health*” | “agricultural soil*” | |
| “soil ecosystem*” | ||
| “heavy metal*” | “agricultural*” | |
| “trace metal*” | “vegetable*” | |
| “food crop*” | ||
| “plant*” | ||
| “antibiotic resistant bacteria*” | “human health*” | “agricultural soil*” |
| “antibiotic resistant genes*” | “agroecosystem*” | |
| “antibiotic resistance” | “agricultural*” | |
| “antibiotic*” | ||
| “microplastic*” | “human health*” | “agricultural soil*” |
| “nanoplastic*” | “agroecosystems*” | |
| “agricultural*” | ||
| Heavy Metals | Micro(nano)plastics | Antibiotic-Resistant Bacteria | |
|---|---|---|---|
| Field Study | 18 | 12 | 10 |
| Desk Study | 25 | 31 | 20 |
| Total | 51 | 52 | 31 |
| HMs | MNPs | ARB | |
|---|---|---|---|
| Sources of contamination | ++ | + | ++ |
| Environmental fate and behaviour | ++ | + | + |
| Human exposure | ++ | +/− | +/− |
| Human health risk | ++ | − | + |
| Status of current soil-related monitoring | ++ | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perković, S.; Paul, C.; Vasić, F.; Helming, K. Human Health and Soil Health Risks from Heavy Metals, Micro(nano)plastics, and Antibiotic Resistant Bacteria in Agricultural Soils. Agronomy 2022, 12, 2945. https://doi.org/10.3390/agronomy12122945
Perković S, Paul C, Vasić F, Helming K. Human Health and Soil Health Risks from Heavy Metals, Micro(nano)plastics, and Antibiotic Resistant Bacteria in Agricultural Soils. Agronomy. 2022; 12(12):2945. https://doi.org/10.3390/agronomy12122945
Chicago/Turabian StylePerković, Stanislava, Carsten Paul, Filip Vasić, and Katharina Helming. 2022. "Human Health and Soil Health Risks from Heavy Metals, Micro(nano)plastics, and Antibiotic Resistant Bacteria in Agricultural Soils" Agronomy 12, no. 12: 2945. https://doi.org/10.3390/agronomy12122945