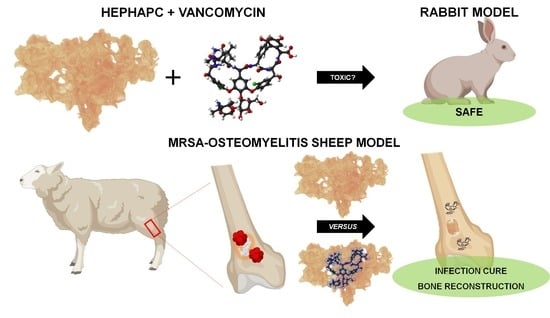
Vancomycin-Loaded, Nanohydroxyapatite-Based Scaffold for Osteomyelitis Treatment: In Vivo Rabbit Toxicological Tests and In Vivo Efficacy Tests in a Sheep Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Drug-Delivery System
2.2. In Vivo GLP Toxicological Tests
2.2.1. Implantation Procedure
2.2.2. Clinical Observations and Mortality
2.2.3. Clinical Pathology—Hematology and Clinical Chemistry
2.2.4. Urinalysis
2.2.5. Terminal Observation
2.2.6. Necropsy
2.2.7. Histological Technique
2.2.8. Histopathology
2.3. In Vivo Sheep Efficacy Testing
2.3.1. Osteomyelitis Induction
Bacterial Strain and Inoculum Preparation
Experimental Procedure
2.3.2. Clinical and Macroscopic Evaluation
2.3.3. Blood Tests
2.3.4. Microbiological Analysis
2.3.5. X-rays
2.3.6. Micro CT Analysis
2.3.7. Histopathological Analysis
2.3.8. Statistical Analysis
3. Results
3.1. In Vivo GLP Toxicology Studies
3.2. In Vivo Sheep Efficacy Testing
3.2.1. Clinical and Macroscopic Evaluation
3.2.2. Blood Tests
3.2.3. Microbiological Analysis
3.2.4. X-rays
3.2.5. Micro CT Analysis
3.2.6. Histopathological Analysis
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurtz, S.M.; Ong, K.L.; Schmier, J.; Mowat, F.; Saleh, K.; Dybvik, E.; Kärrholm, J.; Garellick, G.; Havelin, L.I.; Furnes, O.; et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J. Bone Jt. Surg Am. 2007, 89 (Suppl. 3), 144–151. [Google Scholar]
- Cram, P.; Lu, X.; Kates, S.L.; Singh, J.A.; Li, Y.; Wolf, B.R. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA 2012, 308, 1227–1236. [Google Scholar] [CrossRef]
- Loomba, P.S.; Taneja, J.; Mishra, B. Methicillin and Vancomycin Resistant S. aureus in Hospitalized Patients. J. Glob. Infect. Dis. 2010, 2, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2011, 84, 1027–1033. [Google Scholar] [PubMed]
- Cierny, G., 3rd; Mader, J.; Penninck, J. A clinical staging system for adult osteomyelitis. Clin. Orthop. Relat Res. 2003, 7–24. [Google Scholar] [CrossRef]
- Shuford, J.A.; Steckelberg, J.M. Role of oral antimicrobial therapy in the management of osteomyelitis. Curr. Opin. Infect. Dis. 2003, 16, 515–519. [Google Scholar] [CrossRef]
- Panteli, M.; Puttaswamaiah, R.; Lowenberg, D.W.; Giannoudis, P.V. Malignant transformation in chronic osteomyelitis: Recognition and principles of management. J. Am. Acad Orthop. Surg. 2014, 22, 586–594. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Engelbrecht, H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg 1970, 41, 511–515. [Google Scholar]
- Wahlig, H.; Dingeldein, E.; Bergmann, R.; Reuss, K. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmacokinetic study. J. Bone Jt. Surg. Br. 1978, 60, 270–275. [Google Scholar] [CrossRef]
- Wong, M.W.; Hui, M. Development of gentamicin resistance after gentamicin-PMMA beads for treatment of foot osteomyelitis: Report of two cases. Foot Ankle Int. 2005, 26, 1093–1095. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Muckley, T.; Hofmann, G. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury 2006, 37 (Suppl 2), S95–S104. [Google Scholar] [CrossRef]
- Hanssen, A.D. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 2005, 437, 91–96. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Santos, J.; Monteiro, F. Porous HA Scaffolds for Drug Releasing. Key Eng. Mater. 2005, 284–286, 407–410. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.; Lee, J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The Material Bone: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Gutierres, M.; Hussain, N.S.; Lopes, M.; Afonso, A.; Cabral, A.; Almeida, L.; Santos, J. Histological and scanning electron microscopy analyses of bone/implant interface using the novel Bonelike synthetic bone graft. J. Orthop. Res. 2006, 24, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Gutierres, M.; Hussain, N.S.; Afonso, A.; Almeida, L.; Cabral, T.; Lopes, M.; Santos, J.D. Biological Behaviour of Bonelike® Graft Implanted in the Tibia of Humans. Key Eng. Mater. 2005, 284–286, 1041–1044. [Google Scholar] [CrossRef]
- Gutierres, M.; Lopes, M.; Hussain, N.S.; Lemos, A.; Ferreira, J.; Afonso, A.; Cabral, A.; Almeida, L.; Santos, J. Bone ingrowth in macroporous Bonelike for orthopaedic applications. Acta Biomater. 2008, 4, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.; Ran, J.; Shen, X.; Tong, H. Synthesis and cytocompatibility of collagen/hydroxyapatite nanocomposite scaffold for bone tissue engineering. Polym. Compos. 2016, 37, 81–90. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. Journal of biomedical materials research. Part. B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Lickorish, D.; Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V.; Howlett, C.R. Collagen–hydroxyapatite composite prepared by biomimetic process. J. Biomed. Mater. Res. Part. A 2004, 68, 19–27. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasisekharan, R.; Ernst, S.; Venkataraman, G. On the regulation of fibroblast growth factor activity by heparin-like glycosaminoglycans. Angiogenesis 1997, 1, 45–54. [Google Scholar] [CrossRef]
- Lee, D.-W.; Yun, Y.-P.; Park, K.; Kim, S.E. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone 2012, 50, 974–982. [Google Scholar] [CrossRef]
- Kim, S.E.; Song, S.-H.; Yun, Y.P.; Choi, B.-J.; Kwon, I.K.; Bae, M.S.; Moon, H.-J.; Kwon, Y.-D. The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammation and osteoblast function. Biomaterials 2011, 32, 366–373. [Google Scholar] [CrossRef]
- Coelho, C.C.; Sousa, S.R.; Monteiro, F.J. Heparinized nanohydroxyapatite/collagen granules for controlled release of vancomycin. J. Biomed. Mater. Res. A 2015, 103, 3128–3138. [Google Scholar] [CrossRef]
- Teixeira, S.; Yang, L.; Dijkstra, P.J.; Ferraz, M.P.; Monteiro, F. Heparinized hydroxyapatite/collagen three-dimensional scaffolds for tissue engineering. Journal of materials science. Mater. Med. 2010, 21, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Kinik, H.; Karaduman, M. Cierny-Mader Type III chronic osteomyelitis: The results of patients treated with debridement, irrigation, vancomycin beads and systemic antibiotics. Int. Orthop. 2008, 32, 551–558. [Google Scholar] [CrossRef]
- Gerstein, W.; Colombo, E.; Harji, F. Documented vancomycin-induced severe immune-mediated thrombocytopaenia. BMJ Case Rep. 2018, 2018, 224682. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.F.; Hahn, S.R.; Gonçalves, J.; Fresco, P. Vancomycin therapeutic drug monitoring and population pharmacokinetic models in special patient subpopulations. Pharm. Res. Perspect. 2018, 6, e00420. [Google Scholar] [CrossRef]
- Padrão, T.; Coelho, C.C.; Costa, P.; Alegrete, N.; Monteiro, F.J.; Sousa, S.R. Combining local antibiotic delivery with heparinized nanohydroxyapatite/collagen bone substitute: A novel strategy for osteomyelitis treatment. Mater. Sci. Eng. C 2020, 119, 111329. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Pham, T.X.; Williams, D.L.; Farnsworth, R.W.; Loc-Carrillo, C.M.; Bloebaum, R.D. Model development for determining the efficacy of a combination coating for the prevention of perioperative device related infections: A pilot study. J. Biomed. Mater. Res. B Appl Biomater. 2013, 101, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Rissing, J.P.; Buxton, T.B.; Weinstein, R.S.; Shockley, R.K. Model of experimental chronic osteomyelitis in rats. Infect. Immun. 1985, 47, 581–586. [Google Scholar] [CrossRef]
- Norden, C.W.; Myerowitz, R.L.; Keleti, E. Experimental osteomyelitis due to Staphylococcus aureus or Pseudomonas aeruginosa: A radiographic-pathological correlative analysis. Br. J. Exp. Pathol. 1980, 61, 451–460. [Google Scholar] [PubMed]
- Janko, M.; Dust, F.; Wagner, P.V.; Gurke, R.; Frank, J.; Henrich, D.; Marzi, I.; Verboket, R.D. Local Fixation of Colistin With Fibrin Spray: An in vivo Animal Study for the Therapy of Skin and Soft Tissue Infections. Front. Surg. 2022, 9, 749600. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Lazzarini, L.; Mader, J.T.; Calhoun, J.H. Osteomyelitis in long bones. J. Bone Jt. Surg. Am. 2004, 86, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sudo, A.; Komlev, V.S.; Barinov, S.M.; Uchida, A. High release of antibiotic from a novel hydroxyapatite with bimodal pore size distribution. J. Biomed. Mater. Res. Part. B-Appl. Biomater. 2004, 70, 332–339. [Google Scholar] [CrossRef]
- Ferraz, M.; Mateus, A.; Sousa, J.; Monteiro, F. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. Part. A 2007, 81, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.; Fini, M.; Giavaresi, G.; Giardino, R. Sheep model in orthopedic research: A literature review. Comp. Med. 2001, 51, 292–299. [Google Scholar] [PubMed]
- Nuss, K.; Auer, J.A.; Boos, A.; von Rechenberg, B. An animal model in sheep for biocompatibility testing of biomaterials in cancellous bones. BMC Musculoskelet. Disord. 2006, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, P.F.; Morr, S.; Bogunovic, L.; Kim, A.D.; Park, B.; Lane, J.M. Selection and development of preclinical models in fracture-healing research. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 1), 79–84. [Google Scholar] [CrossRef]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schütz, M.A.; Duda, G.N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef]
- Newman, E.; Turner, A.S.; Wark, J.D. The potential of sheep for the study of osteopenia: Current status and comparison with other animal models. Bone 1995, 16 (Suppl. 4), 277S–284S. [Google Scholar] [CrossRef]
- Stewart, S.; Barr, S.; Engiles, J.; Hickok, N.J.; Shapiro, I.M.; Richardson, D.W.; Parvizi, J.; Schaer, T.P. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: A proof-of-concept study. J. Bone Jt. Surg. Am. 2012, 94, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Haymond, B.S.; Beck, J.P.; Savage, P.B.; Chaudhary, V.; Epperson, R.T.; Kawaguchi, B.; Bloebaum, R.D. In vivo efficacy of a siliconecationic steroid antimicrobial coating to prevent implant-related infection. Biomaterials 2012, 33, 8641–8656. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.F.; Clasper, J.C.; Parker, S.J.; Watkins, P.E. Early intramedullary nailing in an animal model of a heavily contaminated fracture of the tibia. J. Orthop. Res. 2002, 20, 648–653. [Google Scholar] [CrossRef]
- Clasper, J.; Cannon, L.; Stapley, S.; Taylor, V.; Watkins, P. Fluid accumulation and the rapid spread of bacteria in the pathogenesis of external fixator pin track infection. Injury 2001, 32, 377–381. [Google Scholar] [CrossRef]
- Clasper, J.C.; Stapley, S.A.; Bowley, D.M.G.; Kenward, C.E.; Taylor, V.; Watkins, P.E. Spread of infection, in an animal model, after intramedullary nailing of an infected external fixator pin track. J. Orthop. Res. 2001, 19, 155–159. [Google Scholar] [CrossRef] [PubMed]
- von Stechow, D.; Scale, D.; Rauschmann, M.A. Minimizing the surgical approach in patients with spondylitis. Clin. Orthop Relat Res. 2005, 439, 61–67. [Google Scholar] [CrossRef] [PubMed]






| Tissue/Organ | Weight | Fix | Slide | Microscopy |
|---|---|---|---|---|
| Adrenal glands | × | × | × | × |
| Aorta | × | × | × | |
| Brain | × | × | × | × |
| Bone (femur with joint and cartilage) | × | × | × | |
| Cecum | × | × | × | |
| Colon | × | × | × | |
| Duodenum | × | × | × | |
| Epididymides * | × | × | × | × |
| Eyes (incl. optic nerves) * | × | × | × | |
| Heart | × | × | × | × |
| Ileum | × | × | × | |
| Implantation sites (3 from each femur) | × | × | × | |
| Jejunum | × | × | × | |
| Kidneys * | × | × | × | × |
| Lacrimal gland | × | × | × | |
| Larynx | × | × | × | |
| Liver | × | × | × | × |
| Lungs (incl. mainstem bronchi) | × | × | × | |
| Lymph node (iliac, cervical, mesenteric) | × | × | × | |
| Oesophagus | × | × | × | |
| Pancreas | × | × | × | |
| Payers patches | × | × | × | |
| Pituitary | × | × | × | |
| Prostate | × | × | × | × |
| Salivary gland (mandibular, parotid) * | × | × | × | |
| Seminal vesicles | × | × | × | |
| Sciatic nerve | × | × | × | |
| Skeletal muscle | × | × | × | |
| Skin | × | × | × | |
| Spinal cord (at three levels) | × | × | × | |
| Spleen | × | × | × | × |
| Sternum (incl. bone marrow) | × | × | × | |
| Stomach | × | × | × | |
| Testes * | × | × | × | × |
| Thymus | × | × | × | × |
| Thyroids (incl. parathyroid) * | × | × | × | × |
| Tongue | × | × | × | |
| Trachea | × | × | × | |
| Ureters | × | × | × | |
| Urinary bladder | × | × | × | |
| All gross lesions | × | × | × |
| Group | Animal | Rissing Score | Norden Score | ||||
|---|---|---|---|---|---|---|---|
| Initial | At Endpoint | ||||||
| Group A | 1 | 3 | 3 | 4 | 3.33 | 4 | 4 |
| 2 | 3 | 4 | 7 | ||||
| 3 | 3 | 2 | 1 | ||||
| Group B | 4 | 3 | 3 | 4 | 3.67 | 6 | 5.17 |
| 5 | 3 | 4 | 5.5 | ||||
| 6 | 3 | 3 | 4 | ||||
| Group C | 7 | 3 | 3 | 0 | 0 | 0 | 1 |
| 8 | 3 | 0 | 2 | ||||
| 9 | 3 | 0 | 1 | ||||
| Group D | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | ||||
| 12 | 0 | 0 | 0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alegrete, N.; Sousa, S.R.; Padrão, T.; Carvalho, Â.; Lucas, R.; Canadas, R.F.; Lavrador, C.; Alexandre, N.; Gärtner, F.; Monteiro, F.J.; et al. Vancomycin-Loaded, Nanohydroxyapatite-Based Scaffold for Osteomyelitis Treatment: In Vivo Rabbit Toxicological Tests and In Vivo Efficacy Tests in a Sheep Model. Bioengineering 2023, 10, 206. https://doi.org/10.3390/bioengineering10020206
Alegrete N, Sousa SR, Padrão T, Carvalho Â, Lucas R, Canadas RF, Lavrador C, Alexandre N, Gärtner F, Monteiro FJ, et al. Vancomycin-Loaded, Nanohydroxyapatite-Based Scaffold for Osteomyelitis Treatment: In Vivo Rabbit Toxicological Tests and In Vivo Efficacy Tests in a Sheep Model. Bioengineering. 2023; 10(2):206. https://doi.org/10.3390/bioengineering10020206
Chicago/Turabian StyleAlegrete, Nuno, Susana R. Sousa, Tatiana Padrão, Ângela Carvalho, Raquel Lucas, Raphael F. Canadas, Catarina Lavrador, Nuno Alexandre, Fátima Gärtner, Fernando J. Monteiro, and et al. 2023. "Vancomycin-Loaded, Nanohydroxyapatite-Based Scaffold for Osteomyelitis Treatment: In Vivo Rabbit Toxicological Tests and In Vivo Efficacy Tests in a Sheep Model" Bioengineering 10, no. 2: 206. https://doi.org/10.3390/bioengineering10020206