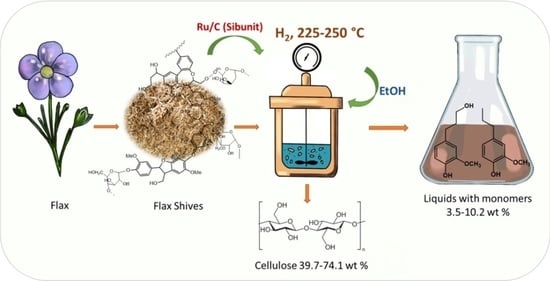
Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Preparation and Characterization
2.2. Catalytic Hydrogenation of Flax Shive in Ethanol
2.3. Characterystics of the Liquid Products of Reductive Catalytic Fractionation of Flax Shive
2.4. Molecular Weight Distribution of the Liquid Products
2.5. Influence of Solvent
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Flax Shive Samples Preparation
3.3. Hydrogenation of Flax Shive
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, K.; Giuseppe, M. Characteristics of lignin from flax shives as affected by extraction conditions. Int. J. Mol. Sci. 2010, 11, 4035–4050. [Google Scholar] [CrossRef] [Green Version]
- Buranov, A.U.; Mazza, G. Fractionation of flax shives with pressurized aqueous ethanol. Ind. Crop. Prod. 2012, 35, 77–87. [Google Scholar] [CrossRef]
- Nuez, L.; Beaugrand, J.; Shah, D.U.; Mayer-Laigle, C.; Bourmaud, A.; D’Arras, P.; Baley, C. The potential of flax shives as reinforcements for injection moulded polypropylene composites. Ind. Crop. Prod. 2020, 148, 112324. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef]
- Bauer, S.; Sorek, H.; Mitchell, V.D.; Ibáñez, A.B.; Wemmer, D.E. Characterization of miscanthus giganteus lignin isolated by ethanol organosolv process under reflux condition. J. Agric. Food Chem. 2012, 60, 8203–8212. [Google Scholar] [CrossRef]
- Sun, Z.; Barta, K. Cleave and couple: Toward fully sustainable catalytic conversion of lignocellulose to value added building blocks and fuels. Chem. Commun. 2018, 54, 7725–7745. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [Green Version]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Taran, O.P.; Gromov, N.V.; Parmon, V.N. CHAPTER 2 Catalytic Processes and Catalyst Development in Biorefining. Sustainable Catalysis for Biorefineries; The Royal Society of Chemistry: London, UK, 2018; pp. 25–64. [Google Scholar]
- Galkin, M.V.; Samec, J.S. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Li, H.; Deng, W.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Lei, H.; Chen, P.; et al. Recent advances in improving lignocellulosic biomass-based bio-oil production. J. Anal. Appl. Pyrolysis 2020, 149, 104845. [Google Scholar] [CrossRef]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation processes: Advances on catalytic transformations of biomass-derived platform chemicals into hydrocarbon fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fang, Z.; Smith, R.L.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Michel, C.; Gallezot, P. Why is ruthenium an efficient catalyst for the aqueous-phase hydrogenation of biosourced carbonyl compounds? ACS Catal. 2015, 5, 4130–4132. [Google Scholar] [CrossRef]
- Bao, D. Dynamics and correlation of platinum-group metals spot prices. Resour. Policy 2020, 68, 101772. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, M.; Fang, Y.; Tan, T. Reductive catalytic fractionation of lignocellulose: When should the catalyst meet depolymerized lignin fragments? Sustain. Energy Fuels 2020, 4, 5588–5594. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Bukhtiyarov, A.V.; Aymonier, C.; Prosvirin, I.P.; Parmon, V.N. Hydrothermal solubilization–hydrolysis–dehydration of cellulose to glucose and 5-hydroxymethylfurfural over solid acid carbon catalysts. Top. Catal. 2018, 61, 1912–1927. [Google Scholar] [CrossRef]
- Taran, O.P.; Polyanskaya, E.M.; Ogorodnikova, O.L.; Descorme, C.; Besson, M.; Parmon, V.N. Sibunit-based catalytic materials for the deep oxidation of organic ecotoxicants in aqueous solution: I. Surface properties of the oxidized sibunit samples. Catal. Ind. 2010, 2, 381–386. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Baryshnikov, S.V.; Chudina, A.I.; Malyar, Y.N.; Sychev, V.V.; Taran, O.P.; Djakovitch, L.; Kuznetsov, B.N. Hydrogenation of abies wood and ethanol-lignin by molecular hydrogen in supercritical ethanol over bifunctional RU/C catalyst. Khimiya Rastitel’nogo Syr’ya 2019, 2, 15–26. [Google Scholar]
- Kuznetsov, B.N.; Sharypov, V.I.; Baryshnikov, S.V.; Miroshnikova, A.V.; Taran, O.P.; Yakovlev, V.A.; Lavrenov, A.V.; Djakovitch, L. Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium. Catal. Today 2020. [Google Scholar] [CrossRef]
- Taran, O.P.; Sharypov, V.I.; Baryshnikov, S.V.; Beregovtsova, N.G.; Miroshnikova, A.V.; Kazachenko, A.S.; Sychev, V.V.; Kuznetsov, B.N. Reductive fractionation of larch in a supercritical ethanol medium in the presence of bifunctional Ru/C catalyst and hydrogen donors. Katal. Promyshlennosti 2020, 20, 127–139. [Google Scholar] [CrossRef]
- Taran, O.P.; Descorme, C.; Polyanskaya, E.M.; Ayusheev, A.B.; Besson, M.; Parmon, V.N. Sibunit-based catalytic materials for the deep oxidation of organic ecotoxicants in aqueous solutions. III: Wet air oxidation of phenol over oxidized carbon and Ru/C catalysts. Catal. Ind. 2013, 5, 164–174. [Google Scholar] [CrossRef]
- Wertheim, G.K.; DiCenzo, S.B. Cluster growth and core-electron binding energies in supported metal clusters. Phys. Rev. B 1988, 37, 844–847. [Google Scholar] [CrossRef]
- Ayusheev, A.B.; Taran, O.P.; Seryak, I.A.; Podyacheva, O.Y.; Descorme, C.; Besson, M.; Kibis, L.S.; Boronin, A.I.; Romanenko, A.I.; Ismagilov, Z.R.; et al. Ruthenium nanoparticles supported on nitrogen-doped carbon nanofibers for the catalytic wet air oxidation of phenol. Appl. Catal. B Environ. 2014, 146, 177–185. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Bykova, M.V.; Ermakov, D.Y.; Khromova, S.A.; Smirnov, A.A.; Lebedev, M.Y.; Yakovlev, V.A. Stabilized Ni-based catalysts for bio-oil hydrotreatment: Reactivity studies using guaiacol. Catal. Today 2014, 220, 21–31. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [Green Version]
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.I.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, J.; Kim, U.-J.; Choi, J.W. Conversion of lignin to phenol-rich oil fraction under supercritical alcohols in the presence of metal catalysts. Energy Fuels 2015, 29, 5154–5163. [Google Scholar] [CrossRef]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef]
- Boonyasuwat, S.; Omotoso, T.; Resasco, D.E.; Crossley, S.P. Conversion of guaiacol over supported ru catalysts. Catal. Lett. 2013, 143, 783–791. [Google Scholar] [CrossRef]
- Omotoso, T.; Boonyasuwat, S.; Crossley, S.P. Understanding the role of TiO2 crystal structure on the enhanced activity and stability of Ru/TiO2 catalysts for the conversion of lignin-derived oxygenates. Green Chem. 2014, 16, 645–652. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Nieto, L.; Jiménez-Barbero, J.; Martínez, Á.T. Structural characterization of guaiacyl-rich lignins in flax (Linum usitatissimum) fibers and shives. J. Agric. Food Chem. 2011, 59, 11088–11099. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, H.; Xiao, L.-P.; Sun, R.-C.; Song, G. Chemodivergent hydrogenolysis of eucalyptus lignin with Ni@ZIF-8 catalyst. Green Chem. 2019, 21, 1498–1504. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. 2015, 51, 13158–13161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, H.; Xiao, L.-P.; Wang, B.; Sun, R.-C.; Song, G. Sequential utilization of bamboo biomass through reductive catalytic fractionation of lignin. Bioresour. Technol. 2019, 285, 121335. [Google Scholar] [CrossRef]
- Ullah, N.; Odda, A.H.; Liang, K.; Kombo, M.A.; Sahar, S.; Ma, L.-B.; Fang, X.-X.; Xu, A.-W. Metal–acid nanoplate-supported ultrafine Ru nanoclusters for efficient catalytic fractionation of lignin into aromatic alcohols. Green Chem. 2019, 21, 2739–2751. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive catalytic fractionation of corn stover lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef] [Green Version]
- Quesada-Medina, J.; López-Cremades, F.J.; Olivares-Carrillo, P. Organosolv extraction of lignin from hydrolyzed almond shells and application of the delta-value theory. Bioresour. Technol. 2010, 101, 8252–8260. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Van den Bosch, S.; Renders, T.; De Boe, T.; Koelewijn, S.F.; Dewaele, A.; Ennaert, T.; Verkinderen, O.; Goderis, B.; Courtin, C.M.; et al. Influence of bio-based solvents on the catalytic reductive fractionation of birch wood. Green Chem. 2015, 17, 5035–5045. [Google Scholar] [CrossRef]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind. Crop. Prod. 2020, 146, 112144. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks: Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef]
- Kürschner, K. Einige kritische erwägungen zur analyse von hölzern. Holz Als Roh Werkst. 1958, 16, 288–297. [Google Scholar] [CrossRef]
- Obolenskaya, A.V.; Elnitskaya, Z.P.; Leonovich, A. Laboratory Works on Chemistry of Wood and Cellulose; Ekologiya: Moscow, Russia, 1991; 320p. [Google Scholar]
- Kürschner, K.; Hoffer, A. Ein neues verfahren zur bestimmung der cellulose in hölzern und zellstoffen a new method for the determination of cellulose in wood and pulps. Technol. Chem. Papier. Zellst. Fabr. 1929, 26, 125–129. [Google Scholar]





| Support | Code | BET Surface Area SBET, m2/g | Pore Volume Vpore, cm3/g | Average Pore Size <dpore>, nm | pHpzc 1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Support | Catalyst | Support | Catalyst | Support | Catalyst | Support | Catalyst | ||
| Sibunit-4 | S4 | 364 | 273 | 0.51 | 0.32 | 5.66 | 4.77 | 7.66 | 8.05 |
| Sibunit-4 oxidized at 400 °C | S400 | 332 | 300 | 0.42 | 0.37 | 5.06 | 5.01 | 6.88 | 7.12 |
| Sibunit-4 oxidized at 450 °C | S450 | 380 | 341/368 2 | 0.53 | 0.50/0.52 2 | 5.66 | 5.88/4.80 2 | 5.33 | 6.89/6.06 2 |
| Sibunit-4 oxidized at 500 °C (grains) | S500g | 287 | 233 | 0.37 | 0.28 | 5.14 | 4.80 | 3.34 | 6.44 |
| Support | Ruthenium Content, wt % | Code | Ru Particle Size, nm 1 | DRu | |||
|---|---|---|---|---|---|---|---|
| dmin | dmax | <dl> | <dS> | ||||
| Sibunit-4, grains 2 | 3 | 3RSg | 0.76 | 3.46 | 1.42 ± 0.02 | 1.71 | 0.77 |
| Sibunit-4 oxidized at 500 °C 2, grains | 3 | 3RS500g | 0.69 | 3.14 | 1.30 ± 0.01 | 1.53 | 0.85 |
| Sibunit-4 oxidized at 400 °C 3 | 3 | 3RS400 | 0.66 | 3.00 | 1.19 ± 0.01 | 1.40 | 0.94 |
| Sibunit-4 oxidized at 450 °C 3 | 3 | 3RS450 | 0.52 | 2.37 | 1.13 ± 0.01 | 1.39 | 0.94 |
| Sibunit-4 oxidized at 450 °C 3 | 1 | 1RS450 | 0.52 | 1.79 | 1.06 ± 0.03 | 1.27 | 1.03 |
| Catalyst. | C | O | Ru | |||
|---|---|---|---|---|---|---|
| at % | wt % | at % | wt % | at % | wt % | |
| 3RS450 | 91.9 | 84.9 | 7.5 | 9.2 | 0.6 | 4.7 |
| 1RS450 | 92.0 | 85.0 | 7.6 | 9.4 | 0.4 | 3.1 |
| No. | Catalyst | Conversion 1, wt % | Liquid Yield | Solid Yield | Gas Yield | Monophenols 2,wt % |
|---|---|---|---|---|---|---|
| 1 | no | 44.1 | 38.5 | 41.0 | 5.6 | 1.52 |
| 2 | 3RS450 | 55.3 | 42.5 | 33.0 | 1.6 | 5.65 |
| 3 | 1RS450 | 52.2 | 44.5 | 41.0 | 7.7 | 4.30 |
| 4 | 3RS500g | 46.7 | 41.0 | 40.0 | 5.7 | 3.54 |
| 5 | 3RS400 | 50.5 | 41.5 | 45.6 | 9.0 | 4.78 |
| 6 | 3RSg | 56.3 | 39.8 | 39.0 | 16.5 | 4.44 |
| 7 | S450 | 39.0 | 34.5 | 41.0 | 4.5 | 2.24 |
| 8 | 3RS450 3 | 43.5 | 21.4 | 56.5 | 16.8 | 3.47 |
| 9 | 3RS450 4 | 87.6 | 31.6 | 12.4 | 37.8 | 5.27 |
| No. | Catalyst | CO wt % | CO2 wt % | CH4 mass % |
|---|---|---|---|---|
| 1 | no | - | 5.6 | - |
| 2 | 3RS450 | 3.6 | 6.8 | 2.2 |
| 3 | 1RS450 | 1.8 | 5.9 | traces |
| 4 | 3RS500g | traces | 5.7 | traces |
| 5 | 3RS400 | 1.4 | 6.6 | 1.0 |
| 6 | 3RSg | 7.4 | 6.5 | 2.6 |
| 7 | Sib-4 (450) | traces | 4.5 | traces |
| 8 | 3RS450 1 | 4.9 | 7.6 | 7.6 |
| 9 | 3RS450 2 | 18.3 | 13.2 | 6.3 |
| No. | Catalysts | Composition of Solid Products | Degree of Delignification, % | Cellulose Yield, wt % | ||
|---|---|---|---|---|---|---|
| Hemicelluloses | Lignin | Cellulose | ||||
| 1 | no | 4.2 | 27.3 | 68.5 | 63.3 | 55.4 |
| 2 | 3RS450 | 5.8 | 15.5 | 79.5 | 83.2 | 51.8 |
| 3 | 1RS450 | 10.4 | 16.3 | 73.3 | 78.1 | 59.3 |
| 4 | 3RS500g | 3.0 | 19.2 | 77.8 | 74.8 | 61.3 |
| 5 | 3RS400 | 11.4 | 14.0 | 74.6 | 79.0 | 67.2 |
| 6 | 3RSg | 5.7 | 25.1 | 69.2 | 66.6 | 55.3 |
| 7 | Sib-4 (450) | 3.1 | 27.6 | 69.3 | 64.9 | 52.9 |
| 8 | 3RS450 1 | 7.8 | 24.7 | 67.5 | 54.9 | 74.1 |
| 9 | 3RS450 2 | 2.1 | 9.2 | 88.7 | 93.1 | 39.7 |
| Substance | Structure | Catalyst | ||||||
|---|---|---|---|---|---|---|---|---|
| Non | 3RS450 | 1RS450 | 3RS500g | 3RS400 | 3RSg | Sib-4 450 | ||
| Guaiacol |  | 3.5 | 1.4 | 1.0 | 3.1 | 0.8 | 2.4 | 2.7 |
| Guaiacylmethane |  | 0.3 | 0.0 | 0.4 | 1.2 | 0.4 | 0.9 | 0.6 |
| Guaiacylethane |  | 5.1 | 5.4 | 3.2 | 7.1 | 4.8 | 7.3 | 5.1 |
| Syringol |  | - | - | - | - | - | 2.4 | - |
| Guaiacylpropane |  | 1.9 | 30.4 | 20.3 | 22.1 | 19.7 | 17.7 | 3.3 |
| Guaiacylpropene |  | 14.7 | 4.8 | 16.9 | 2.6 | 7.5 | 4.2 | 19.9 |
| Syringylethane |  | 0.9 | 1.5 | 0.9 | 1.6 | 1.2 | 1.4 | 1.1 |
| Syringylpropane |  | 0.8 | 7.74 | 6.4 | 5.2 | 5.5 | 4.3 | 1.3 |
| Guaiacylpropanol |  | 2.0 | 6.9 | 10.0 | 2.6 | 21.5 | 7.5 | 1.6 |
| Syringylpropene |  | 2.3 | 0.2 | 1.9 | 0.2 | 0.7 | 0.2 | 3.1 |
| Total monophenol yield per sum of the chromatographed products, % | 31.5 | 61.8 | 62.1 | 49.1 | 64.1 | 47.9 | 38.7 | |
| Substance | Structure | Sample | ||||||
|---|---|---|---|---|---|---|---|---|
| Non | 3RS450 | 1RS450 | 3RS500g | 3RS400 | 3RSg | Sib-4 450 | ||
| Guaiacol |  | 0.36 | 0.55 | 0.30 | 0.77 | 0.24 | 0.49 | 0.40 |
| Guaiacylmethane |  | 0.01 | 0.01 | 0.07 | 0.21 | 0,10 | 0,12 | 0,06 |
| Guaiacylethane |  | 0.21 | 0.88 | 0.36 | 0.73 | 0.64 | 0.17 | 0.31 |
| Guaiacylpropane |  | 0.08 | 4.95 | 2.42 | 2.29 | 2.64 | 1.50 | 0.20 |
| Guaiacylpropene |  | 0.19 | 1.17 | 3.2 | 0.4 | 1.56 | 0.52 | 1.95 |
| Syringylethane |  | 0.04 | 0.24 | 0.11 | 0.16 | 0.16 | 0.30 | 0.18 |
| Syringylpropane |  | 0.03 | 1.26 | 0.76 | 0.54 | 1.12 | 0.36 | 0.08 |
| Guaiacylpropanol |  | 0.08 | 1.09 | 1,19 | 0.27 | 2.87 | 0.64 | 0.10 |
| Syringylpropene |  | 0.14 | 0.06 | 0.34 | 0.02 | 0.15 | 0.03 | 0.24 |
| Total monomer yield, wt % | 1.14 | 10.21 | 8.75 | 5.39 | 9.48 | 4.67 | 3.52 | |
| Total yield of minor methoxyphenol impurities *, wt % | 0.52 | 2.00 | 1.61 | 0.80 | 1.85 | 0.96 | 1.37 | |
| No. | Catalyst | Mn (Da) | Mw (Da) | PD |
|---|---|---|---|---|
| 1 | no | 530 | 1240 | 2.34 |
| 2 | 3RS450 | 430 | 830 | 1.95 |
| 3 | 1RS450 | 450 | 930 | 2.07 |
| 4 | 3RS500g | 450 | 1050 | 2.32 |
| 5 | 3RS400 | 420 | 770 | 1.81 |
| 6 | 3RSg | 460 | 1110 | 2.39 |
| 7 | Sib-4 (450) | 490 | 1070 | 2.20 |
| Ethanol lignin of flax shive * | 890 | 2100 | 2.35 | |
| Substance | Structure | Solvent | ||
|---|---|---|---|---|
| Subcritical Ethanol (225 °C) | Subcritical Isopropanol (225 °C) | Supercritical Ethanol (250 °C) | ||
| Guaiacol |  | 0.55 | 0.19 | 0.83 |
| Guaiacylmethane |  | 0.01 | 0.23 | 0.27 |
| Guaiacylethane |  | 0.88 | 1.93 | 1.28 |
| Syringol- |  | - | - | 0.31 |
| Guaiacylpropane |  | 4.95 | 2.49 | 4.51 |
| Guaiacylpropene |  | 1.17 | 0.27 | - |
| Syringylethane |  | 0.24 | 0.48 | 0.27 |
| Syringylpropane |  | 1.26 | 0.62 | 1.06 |
| Guaiacylpropanol |  | 1.09 | - | 0.85 |
| Syringylpropene |  | 0.06 | - | 0.34 |
| Total yield of alkyphenols. wt % | 10.21 | 6.27 | 9.72 | |
| Total yield of minor methoxyphenol impurities *. wt % | 2.00 | 0.33 | 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Mikhlin, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2021, 11, 42. https://doi.org/10.3390/catal11010042
Kazachenko AS, Tarabanko VE, Miroshnikova AV, Sychev VV, Skripnikov AM, Malyar YN, Mikhlin YL, Baryshnikov SV, Taran OP. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts. 2021; 11(1):42. https://doi.org/10.3390/catal11010042
Chicago/Turabian StyleKazachenko, Aleksandr S., Valery E. Tarabanko, Angelina V. Miroshnikova, Valentin V. Sychev, Andrey M. Skripnikov, Yuriy N. Malyar, Yuriy L. Mikhlin, Sergey V. Baryshnikov, and Oxana P. Taran. 2021. "Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts" Catalysts 11, no. 1: 42. https://doi.org/10.3390/catal11010042