Potential of Native Arbuscular Mycorrhizal Fungi, Rhizobia, and/or Green Compost as Alfalfa (Medicago sativa) Enhancers under Salinity
Abstract
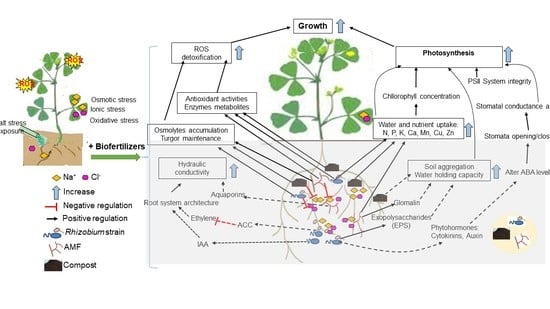
:1. Introduction
2. Materials and Methods
2.1. Plant and Biofertilizer Materials
2.2. Plant Growth Conditions
2.3. Experimental Design and Treatments
2.4. Plant Growth Parameters, Mycorrhizal Assessment, and Nodule Biomass
2.5. Leaf Water Content
2.6. Determination of Nutrient Concentrations in Plants
2.7. Photosynthesis-Related Performance
2.8. Determination of Electrolyte Leakage, Malondialdehyde, and Hydrogen Peroxide Content
2.9. Determination of Proline and Antioxidant Enzymes Concentrations in Plants
2.10. Soil Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of Salinity and Biofertilizers on Symbiotic Development, Growth Parameters, and Shoot Water Status
3.2. Effect of Salinity and Biofertilizers on Shoot Ion Composition
3.3. Biofertilizers Improve the Photosynthesis-Related Performance in Alfalfa Grown under Normal and High-Salinity Conditions
3.4. Biofertilizers Mitigate the Negative Impacts of Salinity by Decreasing the Electrolyte Leakage, Malondialdehyde, and Hydrogen Peroxide Content in Alfalfa Grown under High-Salinity
3.5. Biofertilizers Enhance Osmolytes and Oxidative Defense System in Alfalfa Grown under High Salinity
3.6. Soil Analysis
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA ACC | abscisic acid 1-aminocyclopropane-1-carboxylate |
| AMF | Arbuscular Mycorrhizal Fungi |
| APX | ascorbate peroxidase activity |
| CAT | catalase activity |
| Chl | total chlorophyll content |
| EC | electrical conductivity |
| EE-GPRS | easily extractable glomalin-related soil protein |
| EL | electrolyte leakage |
| EPS | exopolysaccharides |
| Fa | AMF infection frequency |
| Fv/Fm | chlorophyll fluorescence |
| IAA | indole acetic acid |
| gs | stomatal conductance |
| H2O2 | hydrogen peroxide content |
| LWC | leaf water content |
| Ma | AMF infection intensity |
| MDA | malondialdehyde content |
| NDW | nodule dry weight |
| NL | leaf number |
| PH | plant height |
| RDW | root dry weight |
| RL | root length |
| ROS | reactive oxygen species |
| SDW | shoot dry weight |
| SOD | superoxide dismutase activity |
| T-GPRS | total extractable glomalin-related soil protein |
References
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front. Plant Sci. 2020, 11, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Farissi, M.; Bouizgaren, A.; Faghire, M.; Bargaz, A.; Ghoulam, C. Agro-physiological responses of Moroccan alfalfa (Medicago sativa L.) populations to salt stress during germination and early seedling stages. Seed Sci. Technol. 2011, 39, 389–401. [Google Scholar] [CrossRef]
- Baslam, M.; Antolín, M.C.; Gogorcena, Y.; Muñoz, F.; Goicoechea, N. Changes in alfalfa forage quality and stem carbohydrates induced by arbuscular mycorrhizal fungi and elevated atmospheric CO2. Ann. Appl. Biol. 2013, 164, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Baslam, M.; Erice, G.; Goicoechea, N. Impact of arbuscular mycorrhizal fungi (AMF) and atmospheric CO2 concentration on the biomass production and partitioning in the forage legume alfalfa. Symbiosis 2012, 58, 171–181. [Google Scholar] [CrossRef]
- Mbarki, S.; Skalicky, M.; Talbi, O.; Chakraborty, A.; Hnilicka, F.; Hejnak, V.; Zivcak, M.; Brestic, M.; Cerda, A.; Abdelly, C. Performance of Medicago sativa grown in clay soil favored by compost or farmyard manure to mitigate salt stress. Agronomy 2020, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Campanelli, A.; Ruta, C.; Mastro, G.D.; Morone-Fortunato, I. The role of arbuscular mycorrhizal fungi in alleviating salt stress in Medicago sativa L. var. icon. Symbiosis 2013, 59, 65–76. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Meddich, A.; Bechtaoui, N.; Oufdou, K.; Wahbi, S. Effects of arbuscular mycorrhizal fungi and rhizobia symbiosis on the tolerance of Medicago sativa L. to salt stress. Gesunde Pflanz. 2019, 71, 135–146. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 2019, 253, 429–438. [Google Scholar] [CrossRef]
- Timm, C.M.; Carter, K.R.; Carrell, A.A.; Jun, S.; Jawdy, S.S.; Vélez, J.M.; Gunter, L.E.; Yang, Z.; Nookaew, I.; Engle, N.L.; et al. Abiotic stresses shift belowground populus-associated. MSystems 2018, 3, 1–17. [Google Scholar]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef]
- Anli, M.; Baslam, M.; Tahiri, A.I.; Raklami, A.; Boutasknit, A.; Symanczik, S.; Ait-El-Mokhtar, M.B.L.R.; Toubali, S.; Ait-Rahou, Y.; Ait-Chitt, M.; et al. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Baslam, M.; Qaddoury, A.; Goicoechea, N. Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees Struct. Funct. 2014, 28, 161–172. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; Modafar, C.E.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (in)organic adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of salinity stress and microbial inoculations on glomalin production and plant growth parameters of snap bean (Phaseolus vulgaris). Agronomy 2019, 9, 545. [Google Scholar] [CrossRef] [Green Version]
- Jat, R.; Ahlawat, I.P. Direct and residual effect of vermicompost, biofertilizers and phosphorus on soil nutrient dynamics and productivity of chickpea-fodder maize sequence. J. Sustain. Agric. 2006, 28, 97–116. [Google Scholar] [CrossRef]
- Garg, N.; Bharti, A.; Sharma, A.; Bhalla, S. Plant-mycorrhizal and plant-rhizobial interfaces: Underlying mechanisms and their roles in sustainable agroecosystems. In Plant Microbe Interface; Springer International Publishing: Cham, Switzerland, 2019; pp. 27–67. ISBN 9783030198312. [Google Scholar]
- Friesen, M.L.; Porter, S.S.; Stark, S.C.; Von Wettberg, E.J.; Sachs, J.L.; Martinez-Romero, E. Microbially mediated plant functional traits. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Tartoura, K.A.H.; Youssef, S.A.; Tartoura, E.S.A.A. Compost alleviates the negative effects of salinity via up-regulation of antioxidants in Solanum lycopersicum L. plants. Plant Growth Regul. 2014, 74, 299–310. [Google Scholar] [CrossRef]
- Raklami, A.; Tahiri, A.I.; Bechtaoui, N.; Abdelhay, E.G.; Pajuelo, E.; Baslam, M.; Meddich, A.; Oufdou, K. Restoring the plant productivity of heavy metal-contaminated soil using phosphate sludge, marble waste, and beneficial microorganisms. J. Environ. Sci. 2020, 99, 210–221. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; Asli, A.E.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Fakhech, A.; Anli, M.; Ben-Laouane, R.; Boutasknit, A.; Wahbi, S.; Meddich, A. Infectivity of the palm groves arbuscular mycorrhizal fungi under arid and semi-arid climate and its edaphic determinants towards efficient ecological restoration. Rhizosphere 2020, 15, 100220. [Google Scholar] [CrossRef]
- El-Khalloufi, F. Hytotoxicité Induite par les Cyanotoxines: Effets des Microcystines sur la Croissance de Solanum Lycopersicum et sur Medicago Sativa et sa Microflore Rhizosphérique. Ph.D. Thesis, Cadi Ayyad University, Marrakesh, Morocco, 2012. [Google Scholar]
- Alikhani, H.A.; Saleh-Rastin, N.; Antoun, H. Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil 2006, 287, 35–41. [Google Scholar] [CrossRef]
- Lee, V.T.; Matewish, J.M.; Kessler, J.L.; Hyodo, M.; Hayakawa, Y.; Lory, S. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 2007, 65, 1474–1484. [Google Scholar] [CrossRef] [Green Version]
- Bano, N.; Musarrat, J. Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol. 2003, 46, 324–328. [Google Scholar] [CrossRef]
- Hamza, T.A.; Alebejo, A.L. Isolation and characterization of rhizobia from rhizospher and root nodule of cowpea, elephant and lab lab plants. Int. J. Nov. Res. Interdiscip. Stud. 2017, 4, 1–7. [Google Scholar]
- Meddich, A.; Elouaqoudi, F.; Khadra, A.; Bourzik, W. Valorisation des déchets d’origine végétale et industrielle par compostage. Rev. Compos. Matériaux Av. 2016, 26, 451–469. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Mcgonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Olsen, S.R.; Dean, L.A. Phosphorus in: Methods of soil analysis. Am. Soc. Agron. 1965, 9, 920–926. [Google Scholar]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Boursier, P.; Lynch, J.; Lauchli, A.; Epstein, E. Chloride partitioning in leaves of salt-stressed sorghum, maize, wheat and barley. Funct. Plant Biol. 2006, 14, 463. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Madhava Rao, K.V.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Carillo, P.; Mastrolonardo, G.; Nacca, F.; Parisi, D.; Verlotta, A.; Fuggi, A. Nitrogen metabolism in durum wheat under salinity: Accumulation of proline and glycine betaine. Funct. Plant Biol. 2008, 35, 412–426. [Google Scholar] [CrossRef]
- Tejera García, N.A.; Olivera, M.; Iribarne, C.; Lluch, C. Partial purification and characterization of a non-specific acid phosphatase in leaves and root nodules of Phaseolus vulgaris. Plant Physiol. Biochem. 2004, 42, 585–591. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. J. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 566, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Amako, K.; Chen, G.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.C.; Borie, F. Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci. Total Environ. 2008, 406, 154–160. [Google Scholar] [CrossRef]
- Cavagnaro, T.R. Biologically Regulated NutrientSupply Systems. Compost and arbuscular mycorrhizas—A Review. Adv. Agron. 2015, 129, 293–321. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, N.J.; Morieri, G.; Kalsi, G.; Rose, A.; Stiller, J.; Edwards, A.; Xie, F.; Gresshoff, P.M.; Oldroyd, G.E.D.; Downie, J.A.; et al. Rhizobial and mycorrhizal symbioses in Lotus japonicus require lectin nucleotide phosphohydrolase, which acts upstream of calcium signaling. Plant Physiol. 2013, 161, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhangi-Abriz, S.; Torabian, S. Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis 2018, 74, 215–223. [Google Scholar] [CrossRef]
- Liu, H.; Tang, C.; Li, C. The effects of nitrogen form on root morphological and physiological adaptations of maize, white lupin and faba bean under phosphorus deficiency. AoB Plants 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Hao, Z.; Zhou, X.; Jiang, X.; Xu, L.; Wu, S.; Zhao, A.; Zhang, X.; Chen, B. Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza 2018, 28, 285–300. [Google Scholar] [CrossRef]
- Yokoi, S.; Bressan, R.; Hasegawa, P. Salt stress tolerance of plants. JIRCAS Work. Rep. 2002, 23, 25–33. [Google Scholar]
- Rana, M.M.; Takamatsu, T.; Baslam, M.; Kaneko, K.; Itoh, K.; Harada, N.; Sugiyama, T.; Ohnishi, T.; Kinoshita, T.; Takagi, H.; et al. Salt tolerance improvement in rice through efficient SNP marker-assisted selection coupled with speed-breeding. Int. J. Mol. Sci. 2019, 20, 2585. [Google Scholar] [CrossRef] [Green Version]
- Ekinci, M.; Yildirim, E.; Dursun, A.; Turan, M. Mitigation of salt stress in lettuce (Lactuca sativa L. var. Crispa) by seed and foliar 24-epibrassinolide treatments. HortScience 2012, 47, 631–636. [Google Scholar] [CrossRef]
- Salvioli, A.; Zouari, I.; Chalot, M.; Bonfante, P. The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant Biol. 2012, 12. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.G.; Bai, Y.J.; Kong, C.C.; Bian, B.; Xie, Z.H. Synergistic interactions between salt-tolerant rhizobia and arbuscular mycorrhizal fungi on salinity tolerance of Sesbania cannabina plants. J. Plant Growth Regul. 2016, 35, 1098–1107. [Google Scholar] [CrossRef]
- Sieberer, B.J.; Chabaud, M.; Timmers, A.C.; Monin, A.; Fournier, J.; Barker, D.G. A nuclear-targeted cameleon demonstrates intranuclear Ca2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiol. 2009, 151, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Boutasknit, A.; Anli, M.; Tahiri, A.; Raklami, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Ait-Rahou, Y.; Boutaj, H.; Oufdou, K.; Wahbi, S.; et al. Potential effect of horse manure-green waste and olive pomace-green waste composts on physiology and yield of garlic (Allium sativum L.) and soil fertility. Gesunde Pflanz. 2020, 72, 285–295. [Google Scholar] [CrossRef]
- Defez, R.; Andreozzi, A.; Romano, S.; Pocsfalvi, G.; Fiume, I.; Esposito, R.; Angelini, C.; Bianco, C. Bacterial IAA-delivery into medicago root nodules triggers a balanced stimulation of C and N metabolism leading to a biomass increase. Microorganisms 2019, 7, 403. [Google Scholar] [CrossRef] [Green Version]
- Kohler, J.; Caravaca, F.; Roldán, A. An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol. Biochem. 2010, 42, 429–434. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Dong, Y.J.; Wang, W.W.; Hu, G.Q.; Chen, W.F.; Zhuge, Y.P.; Wang, Z.L.; He, M.R. Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J. Soil Sci. Plant Nutr. 2017, 17, 554–569. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.S.; Dietz, K.J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, E.; López-Lefebre, L.R.; García, P.C.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Proline metabolism in response to highest nitrogen dosages in green bean plants (phaseolus vulgaris L. cv. Strike). J. Plant Physiol. 2001, 158, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Hediye Sekmen, A.; Türkan, I.; Takio, S. Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol. Plant. 2007, 131, 399–411. [Google Scholar] [CrossRef]
- Ren, C.G.; Kong, C.C.; Yan, K.; Xie, Z.H. Transcriptome analysis reveals the impact of arbuscular mycorrhizal symbiosis on Sesbania cannabina expose to high salinity. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Eyidogan, F.; Öz, M.T. Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol. Plant. 2007, 29, 485–493. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, X.; Bao, E.; Cao, K.; Zou, Z. Effects of arbuscular mycorrhizal fungi on watermelon growth, elemental uptake, antioxidant, and photosystem II activities and stress-response gene expressions under salinity-alkalinity stresses. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.J.; Yang, H.Y.; Bai, J.P.; Liang, X.Y.; Lou, Y.; Zhang, J.L.; Wang, D.; Zhang, J.L.; Niu, S.Q.; Chen, Y.L. Ultrastructural and physiological responses of potato (Solanum tuberosum L.) plantlets to gradient saline stress. Front. Plant Sci. 2015, 5, 1–14. [Google Scholar] [CrossRef]








| Phosphate Solubilization (mg L−1) | Potassium Solubilization (HD/CD) | Production of Exopolysaccharide (EPS) (mg of CR/OD600) | Production of IAA (µg mL−1) | Nitrogen Fixation |
|---|---|---|---|---|
| 1.55 ± 0.01 | 2.19 ± 0.06 | 156.57 ± 10.60 | 32.37 ± 0.22 | + |
| AMF Infection Frequency (%) | AMF Infection Intensity (%) | Nodules Dry Weight (mg) | Plant Height (cm) | Root Length (cm) | Leaf Number | Leaf Water Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 mM | 120 mM | 0 mM | 120 mM | 0 mM | 120 mM | 0 mM | 120 mM | 0 mM | 120 mM | 0 mM | 120 mM | 0 mM | 120 mM | |
| Ct | - | - | - | - | - | - | 19.7 ± 0.8 g | 13.3 ± 2.2 h | 18.6 ± 0.3 e | 14.0 ± 0.5 d | 9.5 ± 0.3 hi | 6.0 ± 0.6 i | 59.7 ± 1.7 g | 48.9 ± 1.3 h |
| M | 86.6 ± 3.3 a | 66.6 ± 3.3 b | 25.7 ± 5.0 b | 7.0 ± 2.2 c | - | - | 31.5 ± 0.3 ef | 20.1 ± 0.8 g | 24.0 ± 0.5 b | 19.3 ± 0.6 c | 18.0 ± 0.4 fgh | 15.2 ± 1.9 ghi | 71.0 ± 5.0 def | 63.7 ± 3.1 fg |
| R | - | - | - | - | 80.0 ± 0.0 e | 60.0 ± 0.0 f | 33.7 ± 1.5 de | 20.1 ± 1.1 g | 24.3 ± 0.3 b | 19.6 ± 0.6 c | 24.5 ± 0.5 defg | 16.2 ± 2.2 fgh | 71.7 ± 0.2 cdef | 64.9 ± 2.0 fg |
| MR | 85.0 ± 5.0 a | 69.6 ± 2.0 b | 38.0 ± 1.0 a | 6.7 ± 2.8 c | 80.0 ± 0.0 e | 60.0 ± 0.0 f | 36.3 ± 2.1 cd | 23.0 ± 1.4 g | 25.0 ± 1.5 b | 20.3 ± 0.5 c | 32.2 ± 0.7 cde | 26.2 ± 2.1 cdef | 77.9 ± 5.6 bcd | 69.1 ± 2.4 ef |
| C | 33.3 ± 4.0 e | 6.6 ± 0.0 g | 0.6 ± 0.1 e | 0.4 ± 0.2 e | 30.0 ± 0.0 g | 15.0 ± 0.0 i | 39.7 ± 2.7 bc | 21.7 ± 1.7 g | 27.1 ± 2.5 b | 19.0 ± 0.6 c | 29.5 ± 1.9 cde | 22.5 ± 1.5 efg | 76.1 ± 1.4 bcd | 70.9 ± 2.6 def |
| CM | 40.0 ± 5.7 d | 31.0 ± 1.0 e | 5.6 ± 3.1 c | 2.0 ± 1.3 d | 30.0 ± 0.0 g | 20.0 ± 0.0 h | 42.6 ± 1.7 b | 31.2 ± 0.8 ef | 33.3 ± 0.6 a | 20.6 ± 0.3 c | 52.5 ± 4.2 a | 33.0 ± 1.8 cd | 82.1 ± 1.9 ab | 75.6 ± 2.2 bcde |
| CR | 36.3 ± 1.9 d | 16.5 ± 1.8 fg | 0.8 ± 0.1 e | 0.4 ± 0.1 e | 130.0 ± 0.0 b | 90.0 ± 0.0 d | 40.2 ± 1.2 bc | 28.0 ± 0.8 f | 26.3 ± 1.0 b | 18.6 ± 0.3 c | 44.0 ± 3.0 ab | 34.2 ± 3.4 bcd | 80.0 ± 0.7 bc | 76.1 ± 2.7 bcde |
| CMR | 56.5 ± 2.0 c | 38.0 ± 2.8 d | 5.0 ± 2.2 c | 0.5 ± 0.4 e | 140.0 ± 0.0 a | 120.0 ± 0.0 c | 51.0 ± 1.5 a | 33.5 ± 2.1 de | 32.3 ± 1.4 a | 24.0 ± 1.4 b | 51.0 ± 2.9 a | 35.5 ± 3.4 bc | 89.3 ± 0.9 a | 79.3 ± 1.3 bcd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Laouane, R.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S.; et al. Potential of Native Arbuscular Mycorrhizal Fungi, Rhizobia, and/or Green Compost as Alfalfa (Medicago sativa) Enhancers under Salinity. Microorganisms 2020, 8, 1695. https://doi.org/10.3390/microorganisms8111695
Ben-Laouane R, Baslam M, Ait-El-Mokhtar M, Anli M, Boutasknit A, Ait-Rahou Y, Toubali S, Mitsui T, Oufdou K, Wahbi S, et al. Potential of Native Arbuscular Mycorrhizal Fungi, Rhizobia, and/or Green Compost as Alfalfa (Medicago sativa) Enhancers under Salinity. Microorganisms. 2020; 8(11):1695. https://doi.org/10.3390/microorganisms8111695
Chicago/Turabian StyleBen-Laouane, Raja, Marouane Baslam, Mohamed Ait-El-Mokhtar, Mohamed Anli, Abderrahim Boutasknit, Youssef Ait-Rahou, Salma Toubali, Toshiaki Mitsui, Khalid Oufdou, Said Wahbi, and et al. 2020. "Potential of Native Arbuscular Mycorrhizal Fungi, Rhizobia, and/or Green Compost as Alfalfa (Medicago sativa) Enhancers under Salinity" Microorganisms 8, no. 11: 1695. https://doi.org/10.3390/microorganisms8111695