1. Introduction
Date palm (
Phoenix dactylifera L.), is a highly nutrient-rich fruit, originating from the Arabian Peninsula, North Africa, and the Middle East. The worldwide production, utilization and industrialization of dates are increasing continuously [
1]. Date fruit is a rich and cheap source of carbohydrates, proteins, amino acids, and minerals (selenium, potassium, calcium, magnesium, manganese, and iron), dietary fiber, vitamins, carotenoids, and fatty acids [
2,
3]. The biological and therapeutic potential of date flesh and pits demonstrate the presence of polyphenols and flavonoids in both plant tissues [
4].
Dates are an important source of different phytochemicals such as flavonoids, phenolic acids, anthocyanins, tannins, and carotenoids [
5,
6]. Phenolic compounds, volatile compounds, flavonoids (apigenin, luteolin, and quercetin), phytosterols (
β- sitosterol, iso fucosterol, stigmasterol, campesterol), and carotenoids (lutein, neoxanthin,
β-carotene, violoxanthin, antheraxanthin) were also reported in date fruits and pit extracts [
7,
8]. Other authors also documented different polyphenols such as,
p-hydroxybenzoic acid, gallic acid, protocatechic acid, vanillic acid,
o-coumaric acid, caffeic acid, syringic acid, ferulic acid,
p-coumaric acid, 3-caffeoylquinic acid, and 3-
O-caffeoylshikimic acid [
9]. Different date varieties contained soluble phenolic compounds like hydroxybenzoates, hydroxycinnamates and flavonols [
10]. The ripe stage of Khasab, Fard, and Khalas were reported to contain 20, 35, and 63 mg/100 g of phenolic acid, respectively with ferulic acid being the dominant [
11]. Some clinicians also elaborated the low incidence of diabetes, cancer, heart and lung diseases due to judicious use of fruits and vegetables [
4,
5,
6,
8,
12]. Therefore, in developing countries, the use of plant-derived natural products and herbal medicines has significantly increased as alternative solutions to health problems. In this context, the screening, selection and evaluation of pharmacological properties of plant-based natural phytochemicals should be included in the alternative form of health care.
The seeds, stones, and pits of several fruits are used as complementary medicine because of their phytochemical nature that helps to prevent sickness, cure the disease, reduce the side effects, and different kind of stresses. Several reports on date pits showed their functional food properties in dietary treatment [
13], macro- and micronutrients [
14], phenolic acids [
2], as a bread ingredient [
15], as well as protein solubility [
16]. These investigations demonstrate that date pits are not a waste product but a bulk source of natural antioxidants. Antibacterial prospects of various date pits were also reported by various authors against Gram-negative and positive bacteria [
17,
18,
19] and fungi. Furthermore, it was reported that the extraction solvent plays a significant role in determining the phenolic content and these contents are always higher in aqueous extracts in comparison to alcoholic extracts [
20,
21]. The main objective was to evaluate the effect of date pit extracts on Gram-positive (
S. aureus), Gram-negative (
E. coli), bacterial pathogens, and fungi (
Candida albicans). Meanwhile, the total phenolic compounds and flavonoids will be identified from different date pits in order to develop which kind of secondary metabolites are present within the pits and their potential phytotoxicity.
2. Materials and Methods
2.1. Date Pits Material
The fruits of date palm varieties, Abu Maan, Fard, Khalas, Ajwa, Mabroom, Lulu, and Khodari were purchased from Al FOAH COMPANY, Al Saad, Al Ain, Abu Dhabi, United Arab Emirates (UAE). Ripe fruits of best quality, and free from fungal and pest damage were used for this study. Information regarding the cultivars and origin were known based on the information supplied by the supplier. The fruit samples were transported to laboratory and saved at 4 °C until analyzed.
2.2. Date Fruit De-Pitting and Collection of Pits
The pits were removed using a sharp knife at Environmental Engineering lab, University of Sharjah, UAE. The pits were washed with distilled and deionized water and dried at room temperature using two layers of filter papers. The dried pits were crushed and grinded using a grinder (Knifetec 1095 laboratory mill) (10,000 rpm) to make fine powder.
2.3. Organic Solvent Extraction and Treatment Solution Preparation
Different organic solvents, hexane, H2O: Ethanol (EtOH) (1:1), acetone: Water (1:1), ethyl acetate, MeOH: Chloroform (1:1), were used to obtain extracts from different date pits. The date pit powder (20 g) was soaked in selected organic solvent (600 mL) and stirred gently for 24 h at 25 °C. The ingredients attained equilibrium in the organic solvent and then the solvent extract was centrifuged and the supernatant was evaporated to dryness in rotary-evaporators under vacuum at 50 °C. The dry residue was collected, weighed, and stored in air sealed containers at −4 °C until use. The treatment solution (10 mg/mL) was prepared for each pit extract using dimethyl sulfoxide (DMSO). All the treatment solutions were prepared in sterilized conditions by filtering the treatment solutions through Millipore filters (0.45 μm) and stored in the lab fridge at 4 °C. The initial concentration (10 mg/mL) used for testing the antimicrobial activities was further aseptically diluted to determine the minimum inhibitory concentrations (MIC) of the extracts.
2.4. Proximate Analysis
The moisture contents were measured by vacuum oven (method 934.06), protein by Kjeldahl nitrogen (method 920.152), and ash by direct analysis (method 940.26) as per guidelines of the Association of Official Analytical Chemists methods [
22]. The crude protein percentage was measured through the total nitrogen content and multiplied by a factor of 6.25. The lipid contents were evaluated by the method of Bligh and Dyer as described by Hanson and Olley [
23]. Total carbohydrates were calculated as reported previously [
24].
2.5. Dietary Fiber Analysis
The dietary fiber was measured using the AOAC enzymatic gravimetric method 991.43 [
22].
2.6. Total Phenolic Content (Folin–Ciocalteau Assay)
The Folin–Ciocalteau reagent (FCR) is used for determination of total phenolic contents [
25]. The pit extract (40 μL) was thoroughly mixed with FCR (1.8 mL) and left in the lab at room temperature for five min. In this mixture, the sodium bicarbonate (7.5%; 1.2 mL) was added and samples were kept at 24 °C for 60 min. The absorbance was measured later using spectrophotometer at 765 nm and results were expressed as mg gallic acid equivalent (GAE)/100 g sample [
26]. Gallic acid was used as standard in these measurements.
2.7. Total Flavonoids
The flavonoids were determined according to procedure described by Kim et al. [
27]. In the pit extract (1 mL), distilled water (4 mL) was added followed by 5% sodium nitrite solution (0.3 mL) mixing and 10% aluminum chloride solution (0.3 mL). The test tube containing the mixture was incubated for 5 min at ambient temperature and 2 mL NaOH (1 M) was added. Distilled water was supplemented to make the volume 10 mL. The sample was mixed by using vortex and the pink color absorbance was measured at 510 nm. A calibration curve was prepared with catechin and the results were expressed as mg catechin equivalents (CEQ)/100 g sample.
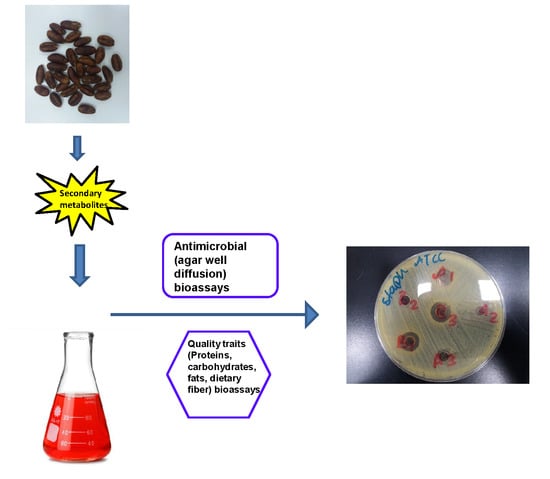
2.8. In Vitro Anti-Microbial Bioassays
The (ATCC) microbial strains were obtained from the stock culture available at the Department of Applied Biology, University of Sharjah, UAE. Three experimental studies were carried out with the main objectives (i) to assess the antimicrobial properties of two popular date varieties (Khalas, Khodari) pit extracts using three organic solvents (hexane, ethyl acetate, H2O: EtOH (1:1) (treated and untreated with UV) on American type culture microbial strains (ATCC), that is, Gram-positive, Staphylococcus aureus (ATCC 29213), Gram-negative, Escherichia coli (ATCC 25922) bacteria and the fungus, Candida albicans (ATCC 66027); (ii) to identify the main phenolic compounds and flavonoids present in the six date palm (Abu Maan, Fard, Khalas, Ajwa, Mabroom, Lulu) pit extracts, (iii) and to evaluate the antibacterial and antifungal activity of these date pit extracts using three different organic solvents (acetone + water (1:1), ethyl acetate, and MeOH + chloroform (1:1).
The antimicrobial activity of pit extract of each variety was first evaluated by the agar ‘well diffusion method’ according to Clinical and Laboratory Standards Institute protocol (CLSI) [
28,
29]. The microorganisms were grown in nutrient agar overnight at 37 °C. A standard suspension with turbidity equal to 0.5 McFarland was prepared on sterile normal saline to give bacterial concentration of 1–2 × 10
8 CFU/mL. Sterile cotton swabs were dipped in the appropriate test organism suspension and evenly streaked over the entire surface of agar plate to obtain semi confluent growths. Six wells (6 mm) per plate were made with the reverse side of the sterilized micropipette tips. Each well was filled with 50 μL of the respective filter-sterilized pit extract using a micropipette. DMSO was used as the negative control. The experiment was conducted under sterilized conditions in a laminar air flow cabinet and then the plates were incubated at 37 °C for 24 h. Standard antibiotics such as amikacin (30 µg) was used as a positive control for
S. aureus and
E. coli and fluconazole (25 µg) was used for
C. albicans. Each extract was analyzed in triplicate. The presence of inhibition zones was observed and their size was measured in mm [
28,
29].
2.9. Minimum Inhibitory Concentration
The MIC is lowest concentration of a antimicrobial agent that can inhibit the visible growth (turbidity) of test microorganisms after overnight incubation. The MICs was evaluated for all date pit extracts using both the micro dilution method and the spectrophotometric assay according to CLSI [
28,
29].
2.10. Statistical Analysis
General Linear Models (GLM) procedure of SPSS software (version 24.0) for Windows (SPSS Inc., Chicago, IL, USA) was used to perform analysis of variance (ANOVA). Data were presented as mean ± standard error and differences among treatment means were compared using Tukey’s HSD test at P < 0.05 significance level.
4. Discussion
Dietary fiber (D.F.) varies between date pits and was in the range of 68.04–75.5% while the highest DF was observed in Abu Maan and the lowest in Mabroom. Dietary fibers are comprised of different plant-based substances that escape the digestion during their transit through the upper gastrointestinal tract [
30,
31]. The total DF in different date varieties ranges from 6.5–11.5% which shows variation with respect to variety and climate [
32]. The polysaccharides in dates comprised of
β-glucan, arabinoxylans, and cellulose. DF has important therapeutic benefits such as anti-diabetic and anti-obesity effects and exhibits a protective effect to maintain a good gut health [
33,
34]. Moreover, important functional properties which are needed for application in the food industry, for example, water-holding, oil-holding, and emulsifying and/or gel formation have also been demonstrated by DF. The desirable properties and stable emulsion-based food products can be developed by incorporating DF in various food products. Mrabet et al., [
35] attempted to extract and analyze dietary fiber from Tunisian secondary date fruit varieties and reported that the content of dietary fiber ranged between 4.7 and 7 g/100 g, thus giving strong indication that the date DF can be utilized as additives in fiber-enriched food [
36]. Furthermore, the dietary fiber is well-known for absorption of cholesterol and declines its availability to the human body and thus renders many health benefits including preventing cardiovascular diseases. Furthermore, the insoluble fraction of the dietary fibers provides a bulking effect and helps in the generation of short chain fatty acids in the intestine during the fermentation [
37]. Health and nutrition specialists agree on the daily consumption of DF, but a recommended daily intake has not been set. However, a daily intake of 25 g/day has been established by well-known nutrition experts. Meanwhile, soluble and insoluble fibers possess different health benefits and consequently more research will further enhance the current knowledge about the quantity and quality profiles of date pits.
Ajwa showed the highest phenolic compounds followed by Khalas. Similarly, Mansouri et al. [
38], also reported a similar quantity of phenolic compounds in Algerian date fruits, except for Kharak date fruits that was significantly higher than for the rest of the varieties. Wu et al. [
39] documented the phenolic compounds in the range of 572–661 mg gallic acid equivalents/00 g fresh weight. Ajwa dates were also analyzed for the total phenolic content which varied from 245–455 mg/100 g. However, it was reported that the extraction procedure and organic solvent might interfere in the extraction of polyphenols. Other researchers documented that the contents are always higher in aqueous extract in comparison to alcoholic extracts [
20,
21]. Various levels of polyphenols like,
p-hydroxybenzoic acid, gallic acid, protocatechic acid, vanillic acid,
o-coumaric acid, caffeic acid, syringic acid, ferulic acid,
p-coumaric acid, 3-caffeoylquinic acid, and 3-
O-caffeoylshikimic acid have been identified in dates [
9]. Different date varieties contained phytochemicals like hydroxybenzoates, hydroxycinnamates, and flavonols [
10]. The ripe stages of Khasab, Fard, and Khalas were reported to contain 20, 35 and 63 mg/100 g of phenolic acid, respectively [
11]. The 5-
O-caffeoylshikimic acid and its isomers like isodactyliferic acid and neodactyliferic acid were reported from Algerian dates (Deglet Noor) [
38].
A similar pattern of variation was found regarding flavonoid contents that were the highest in Ajwa and the lowest in Fard (
Table 2). The difference between flavonoids among different date varieties pits was significant. It was reported that Ajwa contain rutin (0.65–0.85 mg/100 g), catechin (0.73 mg/100 g), and caffeic acid (0.57–1.84 mg/100) [
20,
21]. The phenolic content of Ajwa varied according to their ripening stage (10 mg/100 g–290 mg/100 g). The high polyphenols content is reported in Ajwa at the kimri stage (290 mg/100 g) followed by khalal (150 mg/100 g), rutab (20 mg/100 g) and lowest amount was found in the tamr (10 mg/100 g) stage [
6]. Date fruit displays considerable decrease in polyphenols with the advancement of ripening and increase in its concentration is only seen when the fruit underwent physical damage and microbial infection [
40].
Exploring the antibacterial activities of date pits, several authors also reported the presence of polyphenols, alkaloids, flavonoids, tannins, and steroids that work towards the combined action as antioxidants. These secondary metabolites can serve as potent natural products and phytochemicals and significantly retard the microbial growth, proliferation and infection [
41]. These compounds may act individually as a biologically active compound or provide a synergistic effect to achieve antibacterial properties [
41]. Similarly, in another study, the methanolic extract of Ajwa had antibacterial activity against
E. coli,
Bacillus cereus,
S. aureus, and
Serratia marcescens [
42]. The antibacterial, antifungal and anti-viral activity of extracts obtained from medicinal plants normally depends upon organic solvent, target organism, and plant parts used. Shakiba et al. reported that among all the pits extract tested, the MeOH: CFM extract was more potent at inhibiting the microbial activity. There was no inhibition zone observed for
E. coli (PTCC 1270),
E. coli (PTCC 1399), and
S. marcescens for the methanol extract of Mazafati. However, the same extract inhibited the
E. coli (PTCC 1330) [
43]. This shows that the antibacterial properties of the methanolic extract of the same date palm variety could also possibly be selective against certain strains of bacteria [
43]. Khatami et al. [
44] reported that date pit aqueous extract treated with silver nanoparticles (AgNPs) decreased the
Rhizoctonia solani (AG2_2) population at 25 µg/mL while it also significantly reduced the
Klebsiella pneumonia (PCI 602), and
Acinetobacter baumannii (ATCC 19606). In another study, the acetone and methanol extracts of three date pits showed antibacterial activity against
Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Shigella flexeneri, Staphylococcus aureus, and
Streptococcus pyogenes,. However, aqueous extract had poor phytotoxic impact against all tested microbial pathogens and a negligible effect on
P. aeruginosa. Meanwhile, pits extracts were found to be more effective than leaf extracts [
18]. Earlier, it was reported that methanol and acetone extracts of the
P. dactylifera pits moderately inhibited the growth of Gram-positive and Gram-negative bacteria [
45]. Another study conducted in Iran on four different date pits demonstrated that all pit extracts showed inhibitory effect against
Staphylococcus aureus, but not against
Escherichia coli. Minimum inhibitory concentration and minimum bactericidal concentration of the extracts ranged from 1.56–3.125 and 3.125–12.5 mg mL
−1 for
Staphylococcus aureus, respectively [
46]. It was reported that the phytotoxicity of date pits is mainly due to the presence of secondary metabolites such as cinnamic acids, flavonoid glycosides and flavanols [
44,
45,
46].
Several researchers documented that the date fruit possesses strong antiviral, antibacterial, and antifungal potential [
45]. The ethanol and water extracts of Egyptian date fruits had strong antimicrobial activity against five pathogenic bacterial strains and it was found that the water extracts of date fruit had strong antimicrobial potential against diarrhea-causing bacteria
Salmonella ssp. and
Shigella spp. [
47]. While studying the antifungal activity of aqueous date fruit extract with amphotericin B (antifungal drug), it was found that the therapeutic index of amphotericin B increased significantly when used together with the extract. Moreover, the cytotoxicity (of red blood cells) otherwise induced by amphotericin B was also prevented by the aqueous date extract [
48]. Natural compounds such as phenolic acids, flavonoids, tannins, alkaloids, and steroids present in the plant extract are mostly responsible for herbicidal [
49,
50,
51], antifungal [
52], and antimicrobial activities [
42]. The natural toxins might act individually as pharmaceutically active compounds or provide synergistic effect to exhibit the antimicrobial properties [
42]. The phytotoxicity of phenolic compounds depends upon the presence of hydroxyl group (number and location) [
53]. Flavonoids also exhibited antimicrobial potential and their mode of action was routed through reduction in activity of nucleic acid synthesis, disturbance in energy metabolism and cytoplasmic membrane functions [
42,
54]. The present study indicates that the extraction of secondary metabolites depends upon the solubility and polarity of organic solvents. It was found that methanol and chloroform could extract the phenolic compounds and flavonoids from date palm pits more than other solvents, and acetone alone did not perform very well. However, the efficiency of organic solvents also depends on the plant species [
55]. However, other researchers have diversified opinions and they documented an aqueous extract, methanol, and acetone for phenolic extraction that varies from each other [
56,
57,
58].