HPMC Hydrogel Formation Mechanisms Unveiled by the Evaluation of the Activation Energy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
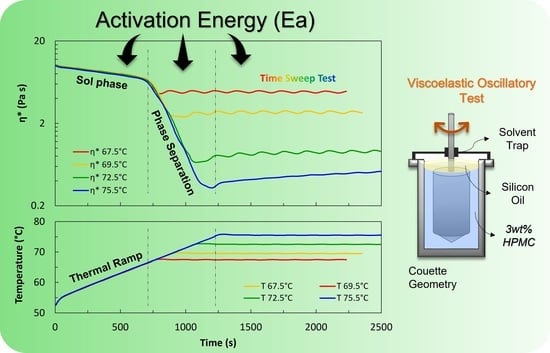
3.1. Time Sweep Tests
3.2. Frequency Sweep Test
3.3. Activation Energy (Ea) Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wüstenberg, T. Cellulose and Cellulose Derivatives in the Food Industry: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Knowlton, J.L.; Pearce, S.E. Handbook of Cosmetic Science & Technology; Elsevier Advanced Technology: Oxford, UK, 2013. [Google Scholar]
- Cinelli, P.; Coltelli, M.B.; Signori, F.; Morganti, P.; Lazzeri, A. Cosmetic packaging to save the environment: Future perspectives. Cosmetics 2019, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Abdul Khalil, H.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A Review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Hon, D.N.-S. Cellulose and its derivatives: Structures, reactions, and medical uses. In Polysaccharides in Medicinal Applications; Routledge: Boca Raton, FL, USA, 2017; pp. 87–105. [Google Scholar]
- Shin, Y.; Kim, D.; Hu, Y.; Kim, Y.; Hong, I.K.; Kim, M.S.; Jung, S. pH-Responsive Succinoglycan-Carboxymethyl Cellulose Hydrogels with Highly Improved Mechanical Strength for Controlled Drug Delivery Systems. Polymers 2021, 13, 3197. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C. Sol-gel behavior of hydroxypropyl methylcellulose (HPMC) in ionic media including drug release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical applications of cellulose ethers and cellulose ether esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef] [PubMed]
- Brumaud, C.; Baumann, R.; Schmitz, M.; Radler, M.; Roussel, N. Cellulose ethers and yield stress of cement pastes. Cem. Concr. Res. 2014, 55, 14–21. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Kiesewetter, R.; Kaufmann, J.; Baumann, R.; Lura, P. Pore structure of mortars with cellulose ether additions–Mercury intrusion porosimetry study. Cem. Concr. Compos. 2014, 53, 25–34. [Google Scholar] [CrossRef]
- Aliu, A.O.; Guo, J.; Wang, S.; Zhao, X. Hydraulic fracture fluid for gas reservoirs in petroleum engineering applications using sodium carboxy methyl cellulose as gelling agent. J. Nat. Gas Sci. Eng. 2016, 32, 491–500. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M. Recent advances in cellulose and its derivatives for oilfield applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef]
- Bemiller, J. Cellulose and Cellulose-Based Hydrocolloids. In Carbohydrate Chemistry for Food Scientists; Elsevier: Duxford, UK, 2019; Volume 12, pp. 223–240. [Google Scholar]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in) solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef] [Green Version]
- Coughlin, M.L.; Liberman, L.; Ertem, S.P.; Edmund, J.; Bates, F.S.; Lodge, T.P. Methyl cellulose solutions and gels: Fibril formation and gelation properties. Prog. Polym. Sci. 2021, 112, 101324. [Google Scholar] [CrossRef]
- Takahashi, M.; Shimazaki, M.; Yamamoto, J. Thermoreversible gelation and phase separation in aqueous methyl cellulose solutions. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 91–100. [Google Scholar] [CrossRef]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef] [PubMed]
- Basta, A.H.; Lotfy, V.F.; Micky, J.A.; Salem, A.M. Liquid crystal behavior of cellulose nanoparticles-ethyl cellulose composites: Preparation, characterization, and rheology. J. Appl. Polym. Sci. 2021, 138, 50067. [Google Scholar] [CrossRef]
- Carotenuto, C.; Grizzuti, N. Thermoreversible gelation of hydroxypropylcellulose aqueous solutions. Rheol. Acta 2006, 45, 468–473. [Google Scholar] [CrossRef]
- Costanzo, S.; Pasquino, R.; Donato, R.; Grizzuti, N. Effect of polymer concentration and thermal history on the inverse thermogelation of hydroxypropylcellulose aqueous solutions. Polymer 2017, 132, 157–163. [Google Scholar] [CrossRef]
- Lopez, C.G.; Richtering, W. Oscillatory rheology of carboxymethyl cellulose gels: Influence of concentration and pH. Carbohydr. Polym. 2021, 267, 118117. [Google Scholar] [CrossRef]
- Miljković, V.; Gajić, I.; Nikolić, L. Waste Materials as a Resource for Production of CMC Superabsorbent Hydrogel for Sustainable Agriculture. Polymers 2021, 13, 4115. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Cuers, J.; Rinken, M.; Adden, R.; Mischnick, P. Critical investigation of the substituent distribution in the polymer chains of hydroxypropyl methylcelluloses by (LC-) ESI-MS. Anal. Bioanal. Chem. 2013, 405, 9021–9032. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. J. Appl. Polym. Sci. 1979, 24, 1073–1087. [Google Scholar] [CrossRef]
- Haque, A.; Richardson, R.K.; Morris, E.R.; Gidley, M.J.; Caswell, D.C. Thermogelation of methylcellulose. Part II: Effect of hydroxypropyl substituents. Carbohydr. Polym. 1993, 22, 175–186. [Google Scholar] [CrossRef]
- Hussain, S.; Keary, C.; Craig, D. A thermorheological investigation into the gelation and phase separation of hydroxypropyl methylcellulose aqueous systems. Polymer 2002, 43, 5623–5628. [Google Scholar] [CrossRef]
- Bajwa, G.S.; Sammon, C.; Timmins, P.; Melia, C.D. Molecular and mechanical properties of hydroxypropyl methylcellulose solutions during the sol: Gel transition. Polymer 2009, 50, 4571–4576. [Google Scholar] [CrossRef]
- Bodvik, R.; Dedinaite, A.; Karlson, L.; Bergström, M.; Bäverbäck, P.; Pedersen, J.S.; Edwards, K.; Karlsson, G.; Varga, I.; Claesson, P.M. Aggregation and network formation of aqueous methylcellulose and hydroxypropylmethylcellulose solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 162–171. [Google Scholar] [CrossRef]
- Fairclough, J.P.A.; Yu, H.; Kelly, O.; Ryan, A.J.; Sammler, R.L.; Radler, M. Interplay between gelation and phase separation in aqueous solutions of methylcellulose and hydroxypropylmethylcellulose. Langmuir 2012, 28, 10551–10557. [Google Scholar] [CrossRef] [Green Version]
- Shahin, A.; Nicolai, T.; Benyahia, L.; Tassin, J.-F.; Chassenieux, C. Evidence for the coexistence of interpenetrating permanent and transient networks of hydroxypropyl methyl cellulose. Biomacromolecules 2014, 15, 311–318. [Google Scholar] [CrossRef]
- Lodge, T.P.; Maxwell, A.L.; Lott, J.R.; Schmidt, P.W.; McAllister, J.W.; Morozova, S.; Bates, F.S.; Li, Y.; Sammler, R.L. Gelation, phase separation, and fibril formation in aqueous hydroxypropylmethylcellulose solutions. Biomacromolecules 2018, 19, 816–824. [Google Scholar] [CrossRef]
- Perez-Robles, S.; Carotenuto, C.; Minale, M. Effect on the thermo-gelation process of the degree and molar substitution of HPMC polymer hydrogels. Macromol. Symp. 2021, in press. [Google Scholar]






| Slope | T1 67.5 (°C) | T2 69.5 (°C) | T3 72.5 (°C) | T4 75.5 (°C) |
|---|---|---|---|---|
| from ƞ* | −1.73·10−5 | −4.39·10−6 | 4.33·10−5 | 1.79·10−4 |
| from G’ | −1.20·10−5 | 2.34·10−5 | 1.18·10−4 | 2.16·10−4 |
| from G” | −1.87·10−5 | −1.03·10−5 | 7.78·10−6 | 1.02·10−4 |
| Ea/R (K) Sol Phase | Ea/R (K) Phase Separation | Ea/R (K) Time Sweep | |
|---|---|---|---|
| Calculated from η* | 2.85·103 ± 26 | 4.92·104 ± 5.8·102 | T1: 4.27·104 ± 2.4·103 T2: 8.84·104 ± 4.9·103 T3: 8.10·104 ± 4.6·103 T4: −2.32·104 ± 1.7·103 |
| Calculated from G’ | 3.48·103 ± 29 | 5.73·104 ± 5.9·102 | T1: 5.29·104 ± 2.9·103 T2: 1.01·105 ± 5.5·103 T3: 3.69·104 ± 2.2·103 T4: −5.12·104 ± 4.0·103 |
| Calculated from G” | 2.62·103 ± 26 | 4.77·104 ± 6.1·102 | T1: 4.00·104 ± 2.3·103 T2: 8.57·104 ± 4.7·103 T3: 1.02·105 ± 4.6·103 T4: 3.50·104 ± 3.5·103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Robles, S.; Carotenuto, C.; Minale, M. HPMC Hydrogel Formation Mechanisms Unveiled by the Evaluation of the Activation Energy. Polymers 2022, 14, 635. https://doi.org/10.3390/polym14030635
Perez-Robles S, Carotenuto C, Minale M. HPMC Hydrogel Formation Mechanisms Unveiled by the Evaluation of the Activation Energy. Polymers. 2022; 14(3):635. https://doi.org/10.3390/polym14030635
Chicago/Turabian StylePerez-Robles, Saray, Claudia Carotenuto, and Mario Minale. 2022. "HPMC Hydrogel Formation Mechanisms Unveiled by the Evaluation of the Activation Energy" Polymers 14, no. 3: 635. https://doi.org/10.3390/polym14030635