Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects
Abstract
:1. Introduction
1.1. Wound Healing Process and Phases
1.2. Classification of Wound Dressings
1.3. Biologically Active Compounds from Marine Organisms
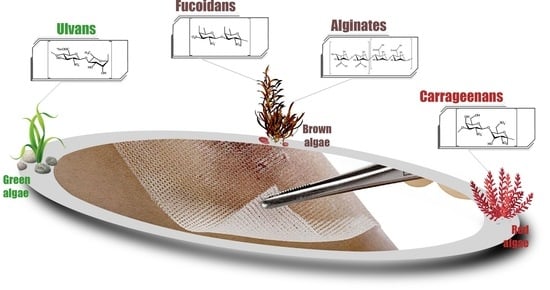
2. Polysaccharides from Marine Algae Used in the Development of Wound Dressings
2.1. Alginates
2.2. Fucoidans
2.3. Carrageenans
2.4. Ulvans
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Basu, S.; Shukla, V. Complications of Wound Healing. In Measurements in Wound Healing; Mani, R., Romanelli, M., Shukla, V., Eds.; Springer: London, UK, 2012; Volume C, pp. 109–144. [Google Scholar]
- Bilal, M.; Iqbal, H.M.N. Marine Seaweed Polysaccharides-Based Engineered Cues for the Modern Biomedical Sector. Mar. Drugs 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabiri, G.; Damstetter, E.; Phillips, T.J. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, R.; Nix, D. Acute and Chronic Wounds, 5th ed.; Mosby: Maryland Heights, MO, USA, 2016; 648p. [Google Scholar]
- Dhivya, S.; Padma, V.V.; Elango, S. Wound dressings—A review. Biomedicine (Taipei) 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.B. The history of wound care. J. Am. Coll. Certif. Wound Spéc. 2011, 3, 65–66. [Google Scholar] [CrossRef] [Green Version]
- Mouës, C.M.; Heule, F.; Legerstee, R.; Hovius, S.E. Five millennia of wound care products--what is new? A literature reviews. Ostomy Wound Manag. 2009, 55, 16–22. [Google Scholar]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. A brief history of wound care. Plast Reconstr Surg. 2006, 117 (Suppl. S7), 6S–11S. [Google Scholar] [CrossRef] [Green Version]
- Aduba, D.C.; Yang, H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering (Basel) 2017, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, T.; Fan, Y. Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, D.-H.; Kim, S.-K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Boil. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y.S. Biologically active substances from marine aquatic organisms—A source of new pharmaceutical substances and medicines. Pac. Med. J. 2010, 2, 5–9. (In Russia) [Google Scholar]
- Kuznetsova, T.A.; Besednova, N.N.; Somova, L.M.; Makarenkova, I.D.; Plekhova, N.G.; Drobot, E.I.; Kovalev, N.N.; Usov, V.V. Experimental evaluation of the effectiveness of wound dressings based on biologically active substances from marine hydrobionts. Russ. J. Mar. Boil. 2016, 42, 427–432. (In Russia) [Google Scholar] [CrossRef]
- Besednova, N.N.; Smolina, T.P.; Andryukov, B.G.; Kuznetsova, T.A.; Mikhailov, V.V.; Zvyagintseva, T.N. Exopolysaccharides of marine bacteria: Prospects for use in medicine. Antibiot. chemother. 2018, 63, 67–78. (In Russia) [Google Scholar]
- Michalak, I.; Chojnacka, K. Algae Biomass: Characteristics and Applications: Towards Algae-Based Products; Chojnacka, K., Wieczorek, P.P., Schroeder, G., Michalak, I., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; 143p. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Chojnacka, K. Marine Algae Extracts. Process, Product and Application; Kim, S.K., Chojnacka, K., Eds.; Wiley: Weinheim, Germany, 2015; Volume 2, 724p. [Google Scholar] [CrossRef]
- Ribeiro, D.M.L.; Júnior, A.R.C.; De Macedo, G.H.R.V.; Chagas, V.L.; Silva, L.D.S.; Cutrim, B.D.S.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; Miranda, R.; et al. Polysaccharide-Based Formulations for Healing of Skin-Related Wound Infections: Lessons from Animal Models and Clinical Trials. Biomolecules 2019, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, M. Wound Healing and Skin Integrity: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2013; 312p. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Thomas, S. A guide to dressing selection. J. Wound Care 1997, 6, 479–482. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Boil. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wind, S.; Kerstein, M.D. Moist wound healing. Dermatol. Nurs. 1996, 8, 204. [Google Scholar]
- Boateng, J.S.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef]
- Krishna, P.S.; Sudha, S.; Reddy, K.A.; Al-Dhabaan, F.A.; Prakasham, R.S.; Charya, M.S. Studies on wound healing potential of red pigment isolated from marine Bacterium Vibrio sp. Saudi J. Boil. Sci. 2019, 26, 723–729. [Google Scholar] [CrossRef]
- Deutsch, C.; Edwards, D.; Myers, P.S. Wound dressings. Br. J. Hosp. Med. 2017, 78, C103–C109. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound Healing Update. Semin. Cutan. Med. Surg. 2012, 31, 33–37. [Google Scholar] [CrossRef]
- Es-Haghi, A.; Mashreghi, M.; Bazaz, M.R.; Homayouni-Tabrizi, M.; Darroudi, M. Fabrication of biopolymer-based nanocomposite wound dressing: Evaluation of wound healing properties and wound microbial load. IET Nanobiotechnol. 2017, 11, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Noël, B.; Charbonneau, L. Modern wound dressings. Praxis (Bern 1994) 2008, 97, 265–268. [Google Scholar] [CrossRef]
- Borkow, G.; Okon-Levy, N.; Gabbay, J. Copper oxide impregnated wound dressing: Biocidal and safety studies. Wounds 2010, 22, 301–310. [Google Scholar]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Min, S.K.; Lee, H.C.; Kwon, Y.S.; Jung, M.H.; Shin, H.S. Spirulina -PCL Nanofiber Wound Dressing to Improve Cutaneous Wound Healing by Enhancing Antioxidative Mechanism. J. Nanomater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Vinnik, Y.; Маркелoва, Н.; Solov’Eva, N.; Shishatskaia, E.; Kuznetsov, M.; Zuev, A. The Current Dressings for Wound Care in the Treatment of Purulent Wounds. Nov. Khirurgii 2015, 23, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrana, N.; Knopf-Marques, H.; Barthes, J. Biomaterials for Organ and Tissue Regeneration: New Technologies and Future Prospects; Woodhead Publishing: Cambridge, UK, 2020; 846p. [Google Scholar]
- Szycher, M. High Performance Biomaterials: A Complete Guide to Medical and Pharmaceutical Applications; CRC Press: Lancaster-Basel, PA, USA, 1991; 824p. [Google Scholar]
- Bhatia, S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer: Gurgaon, India, 2016; 225p. [Google Scholar]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Chen, X.-L.; Zhang, X.-Y.; Zhang, Y.-Z.; Song, X.-Y.; Sun, C.-Y.; Yang, J. Promotion of Wound Healing and Prevention of Frostbite Injury in Rat Skin by Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Mar. Drugs 2020, 18, 48. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.-L.; Zhao, F.; Shi, M.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Chen, X.-L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [Green Version]
- Sahana, T.; Rekha, P. A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro. Int. J. Boil. Macromol. 2019, 131, 10–18. [Google Scholar] [CrossRef]
- Delbarre-Ladrat, C.; Sinquin, C.; Lebellenger, L.; Zykwinska, A.; Colliec-Jouault, S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front. Chem. 2014, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Ovington, L.G. The Evolution of Wound Management. Home Health Care Now 2002, 20, 652–656. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-B.; Qiao, L.-P.; He, H.-L.; Zhang, Q.; Chen, X.-L.; Zhou, W.-Z.; Zhou, B.-C.; Zhang, Y.-Z. Optimization of Fermentation Conditions and Rheological Properties of Exopolysaccharide Produced by Deep-Sea Bacterium Zunongwangia profunda SM-A87. PLoS ONE 2011, 6, e26825. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; Morais, R. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Florez, N.; Gonzalez-Munoz, M.; Ribeiro, D.; Fernandes, E.; Dominguez, H.; Freitas, M. Algae Polysaccharides’ Chemical Characterization and their Role in the Inflammatory Process. Curr. Med. Chem. 2017, 24, 149–175. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Winter, G.D.; Scales, J.T. Effect of Air Drying and Dressings on the Surface of a Wound. Nature 1963, 197, 91–92. [Google Scholar] [CrossRef]
- Hinman, C.D.; Maibach, H. Effect of Air Exposure and Occlusion on Experimental Human Skin Wounds. Nat. 1963, 200, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on Biological Activities and Molecular Characteristics of Sulfated Polysaccharides from Marine Green Algae in Recent Years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, P.; D’auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring Marine Resources for Bioactive Compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabayashi, K.; Ashrafian, H.; Zacharakis, E.; Hasegawa, H.; Kitagawa, Y.; Athanasiou, T.; Darzi, A. Adhesions after abdominal surgery: A systematic review of the incidence, distribution and severity. Surg. Today 2014, 44, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Blunden, G. Biologically active compounds from marine organisms. Phytotherapy Res. 2001, 15, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.S.; Khoo, T.L.; Saad, A.Z.M. Wound bed preparation from a clinical perspective. Indian J. Plast. Surg. 2012, 45, 193–202. [Google Scholar] [CrossRef]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME concept: What have we learned in the past 10 years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Pilcher, M. Wound cleansing: A key player in the implementation of the TIME paradigm. J. Wound Care 2016, 25. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Kordjazi, M.; Shabanpour, B.; Zabihi, E.; Faramarzi, M.A.; Feizi, F.; Gavlighi, H.A.; Feghhi, M.A.; Hosseini, S.A. Sulfated Polysaccharides Purified from Two Species of Padina Improve Collagen and Epidermis Formation in the Rat. Int. J. Mol. Cell. Med. 2013, 2, 156–163. [Google Scholar] [PubMed]
- Pan, H.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.Z.; Li, X. Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater. Sci. Eng. C 2019, 105, 110118. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, K.; Lopez, N. Hydrogel dressings and their application in burn wound care. Br. J. Community Nurs. 2018, 23, S24–S27. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules 2018, 19, 980–988. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Anil, S.; Rao, S.; Bhatnagar, I.; Kim, S.K. Sulfated Polysaccharides from Macroalgae for Bone Tissue Regeneration. Curr. Pharm. Des. 2019, 25, 1200–1209. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Malyarenko, O.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from Brown Alga Fucus evanescens: Structure and Biological Activity. Front. Mar. Sci. 2016, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Boil. Eng. 2020, 14, 1–22. [Google Scholar] [CrossRef]
- Kaiser, D.; Hafner, J.; Mayer, D.; French, L.E.; Läuchli, S. Alginate dressing and polyurethane film versus paraffin gauze in the treatment of split-thickness skin graft donor sites: A randomized controlled pilot study. Adv. Ski. Wound Care 2013, 26, 67–73. [Google Scholar] [CrossRef]
- Williams, C. Kaltostat. Br. J. Nurs. 1994, 3, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Straccia, M.C.; D’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate Hydrogels Coated with Chitosan for Wound Dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [Green Version]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Boateng, J.S.; Burgos-Amador, R.; Okeke, O.; Pawar, H. Composite alginate and gelatin-based bio-polymeric wafers containing silver sulfadiazine for wound healing. Int. J. Boil. Macromol. 2015, 79, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Pawar, H.V.; Boateng, J.S.; Ayensu, I.; Tetteh, J. Multifunctional Medicated Lyophilised Wafer Dressing for Effective Chronic Wound Healing. J. Pharm. Sci. 2014, 103, 1720–1733. [Google Scholar] [CrossRef]
- Murakami, K.; Ishihara, M.; Aoki, H.; Nakamura, S.; Nakamura, S.-I.; Yanagibayashi, S.; Takikawa, M.; Kishimoto, S.; Yokoe, H.; Kiyosawa, T.; et al. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with hydrosheets composed of chitin/chitosan, fucoidan, and alginate as wound dressings. Wound Repair Regen. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Williams, C. Role of CarboFlex in the nursing management of wound odour. Br. J. Nurs. 2001, 10, 122–125. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, I.K.; Kim, Y.S.; Kim, H.J.; Moon, H.; Mueller, S.; Jeong, Y.-I. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater. Res. 2016, 20, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S. Alginate dressings in surgery and wound management—Part 1. J. Wound Care 2000, 9, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.E.; Chircov, C.; Stoica, A.E. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Pek, C.H.; Por, Y.C.; Lim, G.J.S. Biobrane dressing for paediatric burns in Singapore: A retrospective review. Singap. Med. J. 2018, 59, 360–365. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.; Kimble, R.; Stockton, K. Prospective randomised controlled trial of Algisite™ M, Cuticerin™, and Sorbact® as donor site dressings in paediatric split-thickness skin grafts. Burn. Trauma 2018, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S. Alginate dressings in surgery and wound management: Part 2. J. Wound Care 2000, 9, 115–119. [Google Scholar] [CrossRef]
- Totty, J.P.; Bua, N.; Smith, G.; Harwood, A.; Carradice, D.; Wallace, T.; Chetter, I. Dialkylcarbamoyl chloride (DACC)-coated dressings in the management and prevention of wound infection: A systematic review. J. Wound Care 2017, 26, 107–114. [Google Scholar] [CrossRef]
- Thomas, S. Alginate dressings in surgery and wound management: Part 3. J. Wound Care 2000, 9, 163–166. [Google Scholar] [CrossRef]
- Hiro, M.E.; Pierpont, Y.N.; Ko, F.; Wright, T.E.; Robson, M.C.; Payne, W.G. Comparative Evaluation of Silver-Containing Antimicrobial Dressings on In Vitro and In Vivo Processes of Wound Healing. Eplasty 2012, 12, e48. [Google Scholar]
- Pires, A.L.R.; Motta, L.D.A.; Dias, A.M.; De Sousa, H.C.; Moraes, Â.M.; Braga, M.E.M. Towards wound dressings with improved properties: Effects of poly(dimethylsiloxane) on chitosan-alginate films loaded with thymol and beta-carotene. Mater. Sci. Eng. C 2018, 93, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.-F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers (Basel) 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catanzano, O.; D’Esposito, V.; Acierno, S.; Ambrosio, M.; De Caro, C.; Avagliano, C.; Russo, P.; Russo, R.; Miro, A.; Ungaro, F.; et al. Alginate–hyaluronan composite hydrogels accelerate wound healing process. Carbohydr. Polym. 2015, 131, 407–414. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Gong, R.H.; Zhou, F.-L. Electrospun sodium alginate/polyethylene oxide fibers and nanocoated yarns. Int. J. Polym. Sci. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Jayakumar, R.; Kumar, P.S.; Mohandas, A.; Lakshmanan, V.-K.; Biswas, R.; Pt, S.K.; Raja, B. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Boil. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded Silk fibroin/Sodium alginate (SF/SA) blend films. Int. J. Boil. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. The Role of the Pharmacist in Patient Care: Achieving High Quality, Cost-Effective and Accessible Healthcare Through a Team-Based, Patient-Centered Approach; Universal-Publishers: Boca Raton, FL, USA, 2020; 690p. [Google Scholar]
- ALGICELL®. Available online: https://www.woundsource.com/product/algicell-alginate-wound-dressings (accessed on 7 June 2020).
- ALGISIGHT™. Available online: https://www.woundsource.com/product/algisite-m-calcium-alginate-dressing (accessed on 7 June 2020).
- AMERX™. Available online: https://www.woundsource.com/product/amerx-calcium-alginate-dressings (accessed on 7 June 2020).
- Biatain®Alginate. Available online: https://www.coloplast.ca/biatain-alginate-en-ca.aspx (accessed on 8 June 2020).
- CovaWound™ Alginate. Available online: https://www.woundsource.com/product/covawound-alginate-alginate-dressing (accessed on 6 June 2020).
- ExcelGinate. Available online: https://www.woundsource.com/product/excelginate (accessed on 17 May 2020).
- Fibracol™ Plus Collagen Wound Dressing with Alginate. Available online: http://www.woundsource.com/product/fibracol-plus-collagen-wound-dressing-alginate (accessed on 17 May 2020).
- GEMCORE360°™ Reinforced Alginate Wound Dressings. Available online: https://www.woundsource.com/product/gemcore360-reinforced-alginate-wound-dressings (accessed on 17 May 2020).
- Kalginate™ Thin. Available online: https://www.woundsource.com/product/kalginate-thin (accessed on 17 May 2020).
- 3M™ Tegaderm™ High Integrity и 3M™ Tegaderm™ High Gelling Alginate Dressings. Available online: https://www.woundsource.com/product/3m-tegaderm-high-integrity-and-3m-tegaderm-high-gelling-alginate-dressings (accessed on 6 June 2020).
- Jenks, M.; Craig, J.; Green, W.; Hewitt, N.; Arber, M.; Sims, A. Tegaderm CHG IV Securement Dressing for Central Venous and Arterial Catheter Insertion Sites: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy 2016, 14, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Choi, S.-H.; Park, S.-J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.-M.; Ku, S.-K.; Song, C.-H. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs 2017, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; Ulber, R. Fucoidans: Downstream Processes and Recent Applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef] [Green Version]
- Benbow, N.L.; Webber, J.L.; Karpiniec, S.; Krasowska, M.; Ferri, J.K.; Beattie, D.A. The influence of polyanion molecular weight on polyelectrolyte multilayers at surfaces: Protein adsorption and protein—polysaccharide complexation/stripping on natural polysaccharide films on solid supports. Phys. Chem. Chem. Phys. 2017, 19, 23790–23801. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of Hepatocyte Growth Factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B 2010, 86, 588–610. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.Y.; Wang, H.; Tan, Y. [Role of Hepatocyte Growth Factor in Wound Repair]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018, 40, 822–826. [Google Scholar] [CrossRef]
- O’Leary, R.; Rerek, M.; Wood, E.J. Fucoidan modulates the effect of transforming growth factor (TGF)-beta1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Boil. Pharm. Bull. 2004, 27, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Charboneau, A.J.; Delaney, J.P.; Beilman, G. Fucoidans inhibit the formation of post-operative abdominal adhesions in a rat model [published correction appears in PLoS One. 2019; 14(1): e0211371]. PLoS ONE 2018, 13, e0207797. [Google Scholar] [CrossRef]
- Cashman, J.D.; Kennah, E.; Shuto, A.; Winternitz, C.; Springate, C.M. Fucoidan Film Safely Inhibits Surgical Adhesions in a Rat Model. J. Surg. Res. 2011, 171, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.-N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Xue, C.; Tang, Q.; Li, D.; Wu, X.; Wang, J. Isolation and characterization of a sea cucumber fucoidan-utilizing marine bacterium. Lett. Appl. Microbiol. 2010, 50, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [Green Version]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination To Target Herpes Simplex Virus 2 and Human Papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [Green Version]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018, 9, 3881. [Google Scholar] [CrossRef]
- Zepon, K.M.; Martins, M.M.; Marques, M.S.; Heckler, J.M.; Morisso, F.D.P.; Moreira, M.G.; Ziulkoski, A.L.; Kanis, L.A. Smart wound dressing based on κ–carrageenan/locust bean gum/cranberry extract for monitoring bacterial infections. Carbohydr. Polym. 2019, 206, 362–370. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer-based carrageenan blends and composites. Int. J. Boil. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review. J. Mar. Sci. Eng. 2020, 8, 481. [Google Scholar] [CrossRef]
- Radiation Effects in Polymeric Materials; Kumar, V.; Chaudhary, B.; Sharma, V.; Verma, K. (Eds.) Springer: Cham, Switzerland, 2019; 412p. [Google Scholar]
- Tytgat, L.; Van Damme, L.; Arevalo, M.D.P.O.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. Int. J. Boil. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Tan, Y.J.; Li, L. A strategy for strong interface bonding by 3D bioprinting of oppositely charged κ-carrageenan and gelatin hydrogels. Carbohydr. Polym. 2018, 198, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered injectable hydrogels from kappa-carrageenan and two-dimensional nanosilicates for wound healing application. Acta Biomater. 2018, 70, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Singh, A.; Singh, R. Polyvinyl pyrrolidone/carrageenan blend hydrogels with nanosilver prepared by gamma radiation for use as an antimicrobial wound dressing. J. Biomater. Sci. Polym. Ed. 2015, 26, 1269–1285. [Google Scholar] [CrossRef]
- El-Fawal, G.F.; Yassin, A.M.; El-Deeb, N.M. The Novelty in Fabrication of Poly Vinyl Alcohol/κ-Carrageenan Hydrogel with Lactobacillus bulgaricus Extract as Anti-inflammatory Wound Dressing Agent. AAPS PharmSciTech 2016, 18, 1605–1616. [Google Scholar] [CrossRef]
- Schaude, C.; Fröhlich, E.; Meindl, C.; Attard, J.; Binder, B.; Mohr, G.J. The Development of Indicator Cotton Swabs for the Detection of pH in Wounds. Sensors 2017, 17, 1365. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.V.; Raman, M.; Doble, M. Cyclic β-(1→3) (1→6) glucan/carrageenan hydrogels for wound healing applications. RSC Adv. 2016, 6, 98545–98553. [Google Scholar] [CrossRef]
- Tamayol, A.; Akbari, M.; Zilberman, Y.; Comotto, M.; Lesha, E.; Serex, L.; Bagherifard, S.; Chen, Y.; Fu, G.; Ameri, S.K.; et al. Flexible pH-Sensing Hydrogel Fibers for Epidermal Applications. Adv. Heal. Mater. 2016, 5, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Oh, J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Boil. Macromol. 2016, 82, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kanno, K.; Akiyoshi, K.; Nakatsuka, T.; Watabe, Y.; Yukimura, S.; Ishihara, H.; Shin, N.; Kawasaki, Y.; Yano, D. Biocompatible Hydrogel from a Green Tide-Forming Chlorophyta. J. Sustain. Dev. 2012, 5, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Pinho, E.D.; Neves, N.; Sousa, R.A.; Reis, R.L. Processing ulvan into 2D structures: Cross-linked ulvan membranes as new biomaterials for drug delivery applications. Int. J. Pharm. 2012, 426, 76–81. [Google Scholar] [CrossRef]
- Toskas, G.; Hund, R.-D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva Rigida. Carbohydr. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; De Nys, R.; Glasson, C.R. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Mata, L.; Magnusson, M.; Paul, N.A.; De Nys, R. The intensive land-based production of the green seaweeds Derbesia tenuissima and Ulva ohnoi: Biomass and bioproducts. J. Appl. Phycol. 2016, 28, 365–375. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Sims, I.M.; Carnachan, S.M.; De Nys, R.; Magnusson, M. A cascading biorefinery process targeting sulfated polysaccharides (ulvan) from Ulva ohnoi. Algal Res. 2017, 27, 383–391. [Google Scholar] [CrossRef]
- Nardelli, A.; Chiozzini, V.G.; Braga, E.S.; Chow, F. Integrated multi-trophic farming system between the green seaweed Ulva lactuca, mussel, and fish: A production and bioremediation solution. J. Appl. Phycol. 2018, 31, 847–856. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Rizk, M.Z.; El-Sherbiny, M.; Borai, I.H.; Ezz, M.K.; Aly, H.F.; Matloub, A.A.; Farrag, A.E.R.; Fouad, G.I. Sulphated polysaccharides (SPS) from the green alga ulva fasciata extract modulates liver and kidney function in high fat diet-induced hypercholesterolemic rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 43–55. [Google Scholar]






| Commercial Name | Feature | Benefits | Refs |
|---|---|---|---|
| AlgiCell® Ag (Integra LifeSciences Corp.) | Antimicrobial gel high-strength calcium-alginate dressing with complex silver ion transfer technology (1.4%) | - Consists of a patented mixture of D-mannuronic and L-guluronic acids, which provides good gelation and high moisture resistance. - High absorption capacity - Removing the dressing does not leave any residue of silver coated nylon thread in the wound | [23,29,81,108,109] |
| Derma AlgiCell® (Integra LifeSciences Corp.) | This is a soft, sterile calcium alginate dressing. Consists of a mixture of D-mannuronic and L-guluronic acids. | - absorbs from moderate to a large amount of exudate. Covers or fills the wound cavity. - Easily and painlessly removed during dressings. - Maintains a moist wound environment. | [86,89,108] |
| AlgiCell® (Integra LifeSciences Corp.) | Nonwoven wound dressings, dressings, or fibers. | Upon contact with exudate, these dressings form a wet gel during ion exchange. - High gel strength, painless and atraumatic removal from the wound. - Well maintains moisture in the wound. | [94,99,108] |
| AlgiSite® M (Smith and Nephew, Inc.) | An alginate dressing containing calcium forms a hydrophilic gel upon contact with exudate. | - Helps prevent scab formation and helps reduce wounds. Easy painless removal when changing. - Optimizes gas exchange in the wound bed. | [82,94,106,110] |
| Amerx® (Amerx Health Care Corp.) | Dressings in the form of a sterile, elastic pad containing calcium. | - High absorbency, quickly forms a hydrophilic gel to create and maintain optimal moisture in the wound. Convenient packaging, easy to use. | [84,111] |
| Biatain® Alginate (Coloplast Corp.) | High-performance alginate dressings with high absorbent properties. | - Available in waterproof format. - It has hemostatic properties. - High biocompatibility. - Optimal drainage of wound exudate. | [80,93,112] |
| Cuticerin™ Gauze Dressings (Smith and Nephew, Inc.) | Alginate mesh dressing soaked in neutral hydrophobic euserin ointment, petroleum jelly, paraffin. | - Impregnated soft acetate fibers reduce the risk of granulation tissue growing through the dressing. | [20,94,108] |
| CarboFLEX® (ConvaTec) | Hydrocolloid, sterile, non-adhesive, five-layer dressing for direct contact with the wound, absorption of odors. | - Specially designed to solve control problems associated with unpleasant odors on the wound. | [20,88] |
| Carbonet® (ConvaTec) | A multi-layered, flexible and soft odor-absorbing dressing that is highly adaptable to wound contours | - Specially designed to solve control problems associated with unpleasant odors on the wound. - Forms a soft, hydrophilic, gas-permeable gel upon contact with exudate. | [20,42,80,108] |
| CovaWound™ (Covalon Technologies, Ltd.) | A primary wound dressing made from the calcium salt of alginic acid rich in D-mannuronic acid. | - The dressing follows the contours of the wound and provides a microenvironment that promotes wound healing. | [18,41,42,113] |
| Cutimed® Alginate (Essity) | Hydrogel alginate dressing, has a high absorbency and helps maintain a moist environment in the wound. | - Maintains a moist environment in the wound - Fast gelation upon contact with exudate. High gel stability. Highly absorbent base provides effective drainage of the wound. | [20,22,42,108] |
| DermaGinate™ 12” Rote (DermaRite Industries, LLC) | Calcium-alginate dressing that easily fills the wound bed. | - Forms a calming gel-like consistency upon contact with wound exudate. - Easily and painlessly removed during dressings. | [20,41,92] |
| DermaGinate/Ag™ (DermaRite Industries, LLC) | Silver-alginate dressing. It limits the growth of bacteria in a dressing to reduce the risk of secondary infection of wounds. | - Effectively sorb from moderate to significant volume of exudate. Easily fills a bed of wounds. - Creates a soothing gel-like consistency upon contact with exudate in the wound. - Maintains moisture in wounds. | [42,92] |
| DynaGinate™ (DermaRite Industries, LLC) | A sterile dressing made of calcium alginate, designed to protect the wound and maintain its moist environment. | - It has a high absorption capacity, which is designed to absorb moisture 17 times its own weight. - Easily forms a gel in contact with exudate, maintains wound moisture and speeds up the healing process. | [42,92] |
| ExcelGinate™ (MPM Medical, Inc., USA) | Primary non-woven calcium alginate dressing for partial or full thickness of wounds with moderate or severe drainage. | - Tightly woven, when removed, the integrity of the coating is fully preserved. - Highly absorbent coating properties, absorbs four times its weight. - Can be used on infected wounds. | [20,42,82,114] |
| Fibracol™Plus (Systagenix) | Combination dressing of 90% collagen and 10% alginate. | - Maintains integrity when wet. - Does not stick when removed, does not leave fibers in the wound. - Alginate helps maintain a moist environment in the wound, stimulates the formation of granulation tissue and epithelization. | [20,42,83,115] |
| GEMCORE360° ™ (GEMCO Medical, USA) | Reinforced dressing with calcium alginate in the form of antimicrobial foam soaked in polyhexamethylene biguanide | - Maintains a moist wound environment. - Forms a soft, flexible hydrophilic layer of gel upon contact with exudate. - Active against a wide range of bacteria (including MRSA, MRSE, VRE, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans и Rhodotorula mucilaginosa) | [20,84,116] |
| Kalginate™ Thin (DeRoyal, USA) | Alginate primary dressing of heavy fibers for adsorption of exudate. | - Absorb exudate up to 20 times its weight. - Great for daily dressings. - Forms a soluble sodium gel upon contact with liquid contents in the wound. - Available in coating or turunda options | [49,85,117] |
| KALTOSTAT® Alginate Dressing (ConvaTec, UK) | The alginate dressing forms an absorbent gel-fiber matrix in contact with the liquid. | - Supports a moist wound environment and facilitates atraumatic removal. - Used for infected wounds under the supervision of a medical professional. | [21,42,79] |
| KALTOSTAT® Alginate Rope (ConvaTec, UK) | Forms an absorbing matrix of gel fiber in contact with wound fluid | - Can be used for tamponade with nosebleeds. | [21,42,79] |
| 3M™ Tegaderm™ High Integrity (3M + KCI, USA) | Highly resistant alginate coating containing adsorbent | - Provides highly stable gelation and optimal wet environment. Compatible with 3M dressings. - Increased absorbent properties of the dressing - High comfort for the patient during dressings. | [80,118,119] |
| 3M™ Tegaderm™ High Integrity (3M + KCI, USA) | Provides good gelation | - Forms a mechanically strong gel, plugging the entire cavity of the wound. - High comfort for the patient during dressings. - Compatible with 3M dressings. | [80,118,119] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301. https://doi.org/10.3390/biomedicines8090301
Andryukov BG, Besednova NN, Kuznetsova TA, Zaporozhets TS, Ermakova SP, Zvyagintseva TN, Chingizova EA, Gazha AK, Smolina TP. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines. 2020; 8(9):301. https://doi.org/10.3390/biomedicines8090301
Chicago/Turabian StyleAndryukov, Boris G., Natalya N. Besednova, Tatyana A. Kuznetsova, Tatyana S. Zaporozhets, Svetlana P. Ermakova, Tatyana N. Zvyagintseva, Ekaterina A. Chingizova, Anna K. Gazha, and Tatyana P. Smolina. 2020. "Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects" Biomedicines 8, no. 9: 301. https://doi.org/10.3390/biomedicines8090301