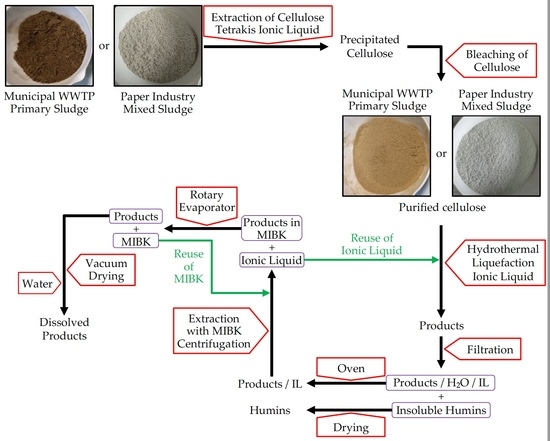
2.4. Bleaching of Precipitate
The procedure of the bleaching of recovered precipitate was based on the literature [
26,
27]. According to these works, hydrogen peroxide is commonly used as a bleaching agent responsible for lignin dissolution. In the first step of the process, a dried batch of recovered precipitate from municipal or industrial sludge was mixed with a solution of hydrogen peroxide (H
2O
2) 8% for the dissolution of lignin and protein. The mixture was stirred 24 h at room temperature. Then, the mixture was filtered. The precipitate was cleaned with distillate water. The content of protein and ashes were determined in both precipitates. The results of the bleaching process are presented in
Table 3.
Proteins were determined after H
2O
2 treatment. As it can be seen in the table, the H
2O
2 solution was able to dissolve and remove 28% and 56% of protein from municipal and industrial precipitates, respectively. Proteins dissolution in H
2O
2 can be explained by the dissociation of H
2O
2 to hydrogen (H
+) and hydroxyl (OH
−) radicals. Then, these oxidative agents readily attacked proteins and decomposed them into soluble amino acids [
28]. However, H
2O
2 did not reduce the quantity of ashes from the precipitate, acid hydrolysis with 0.1 N HCl is expected to do that.
In the second step, both precipitates were hydrolyzed with hydrochloric acid (HCl) 0.1 N during 5 h in an ultrasonic bath. The initial transparent acid dissolution changed color to light brown/yellow. After separation, cleaning and drying of precipitate, ashes were quantified. The acidic treatment was able to remove 70% and 36% of the ashes from municipal and industrial precipitates, respectively. It is noticeable that during the bleaching treatment a loose of carbohydrates was also observed, 15% and 12% from municipal and industrial precipitates, respectively. According to the literature [
26], the hydrolysis also makes isolation of the pure cellulose fibers by hydrolyzing traces of hemicellulose and lignin to simple sugars. In
Table 4 are presented the results of high-performance liquid chromatography (HPLC) analysis of precipitates before and after bleaching. It can be seen in the table that the amount of hemicellulose and lignin reduced after bleaching. However, the amount of cellulose increased for both sludges, more in the case of the industrial sludge, from 32.8 to 42.3%. This increasing can be explained by the growth of the amount of simple sugars, such as glucose, provoked by the treatment with HCl. As the measure of cellulose is obtained from the values of glucose [
29], the results show an increasing amount of cellulose.
Figure 3 presents Fourier Transform Infrared (FTIR) spectra of purified cellulose recovered from municipal sludge (a) and industrial sludge (b). Both spectra present some characteristic absorbances in different frequency regions: 3300 cm
−1 of O–H group, 2900 cm
−1 assigned as the CH
3 and CH
2 stretching vibration of cellulose, 1160 cm
−1 of C–O–C stretching vibration particularly associated with cellulose and the broad peak 1030 cm
−1 of C–O stretching vibration of carbohydrates. All of those peaks demonstrate that purified solids contain cellulose. In
Figure 3a there is also presented the peak at 1650 cm
−1 associated to peptide amide groups of proteins while in
Figure 3b, is absent. That confirms that after bleaching some part of proteins (6.8%) stay in the cellulose recovered from municipal sludge whereas the amount of proteins in the purified cellulose recovered from industrial sludge is too small (1.8%) to be visible in the spectra.
2.5. Cellulose Conversion to Levulinic Acid
The procedure of conversion of cellulose to levulinic acid catalyzed by Brønsted acidic ionic liquid was directly based on a recent work [
3]. In this work, authors have synthesized and tested nine different ionic liquids. The results showed that the acidity of the ionic liquid has the greater importance on the yield of the reaction. The acidity depends on the cation group and on the anion. The [mimC
4SO
3H] [HSO
4] ionic liquid has the higher acidity resulting in its design by the presence of the imidazolium group and by the hydrogen sulfate anion [
3]. In consequence, [mimC
4SO
3H] [HSO
4] was selected and synthesized to convert cellulose to levulinic acid by catalyzed hydrothermal liquefaction.
After the reaction, the reactor was cleaned with deionized water and the products were separated and characterized. The first separation was realized by filtration. Residual solids were abundantly washed with deionized water, dried and weighed. The liquid phase is a mixture of water, ionic liquid and products. Water was evaporated overnight in an oven. Then, the ionic liquid was separated from the resulting liquid mixture by addition of methyl isobutyl ketone (MIBK). Two liquid phases were formed, the upper phase contain the levulinic acid dissolved in the MIBK while the lower phase the ionic liquid. Both phases were separated by centrifugation at 8000 rpm for 5 min. MIBK was evaporated from organic phase at the rotary evaporator at 85 °C and 250 mbar. Resultant products were dried under vacuum and weighed. Finally, products were characterized by HPLC. In
Table 5 are presented the values of the weight of biomass, weight of cellulose contained in the biomass, weight of ionic liquid, and volume of water used in each reaction.
Table 5 also presents the values of weight of soluble in MIBK products, weight and percentage of levulinic acid and finally weight and percentage of humins.
As it was expected, levulinic acid was obtained in each reaction with the three sources of cellulose: pure from provider, from municipal and from industrial sludge. About the commercial cellulose, entries 1–3, it can be observed that with a reaction time of only 2 h, the conversion to levulinic acid was only 18.8%. Increasing the reaction time to 5 h allowed a conversion of 57.8%. This is normal, increasing the reaction time produces an increase in the conversion. However, this increase in conversion reaches a maximum and then decreases, due to the appearance of condensation and recombination reactions of the products. Then, it will be necessary in a future work to confirm this fact, because it is possible that optimized time of reaction was not attained. On the other hand, the decrease of the ratio between the weight of the ionic liquid and the weight of cellulose in the sample, wIL/wCellulose: from 6.67 to 1.88, allowed to increase the conversion to levulinic acid until 81.3%. The effect of the ratio wIL/wCellulose on the conversion to levulinic acid seems to be important. This will be studied in more detail in further work.
The results of conversion to levulinic acid obtained with industrial sludge are less good (entries 4–7). In the four experiments carried out, the conversion to levulinic acid was between 7.1 and 31.0%. The reduction of the conversion is normal since in the samples of industrial sludge there is presence of hemicellulose, lignin, ash and other materials that disturb the reaction of conversion of the cellulose. As it was the case with commercial cellulose (Sigmacell), the decrease in the ratio wIL/wCellulose causes an increase in the conversion to levulinic acid. Indeed, in entries 4 and 7 the ratio is 9.5 and the conversions are 7.1 and 18.4%, respectively. In entries 5 and 6 the ratio is 7.0 and conversions are 10.5 and 31.0%, respectively. However, there is a disparity between the results obtained with the same ratio. There is another variable that seems to be important: the water to cellulose ratio, wWater/wCellulose. Indeed, in entries 4 and 7 the wWater/wCellulose ratios were 64.3 and 47.4, respectively. On the other hand, in entries 5 and 6 the wWater/wCellulose ratios were 47.4 and 42.9, respectively. The decrease in the wWater/wCellulose ratio causes a consequent increase in the conversion to levulinic acid. These trends will have to be confirmed in subsequent work.
In the case of municipal sludge, entries 8–10, the results of conversion to levulinic acid were quite similar: between 11.3 and 25.0%. Again, impurities in the samples were responsible of the decrease of the conversion to levulinic acid. However, with municipal sludge, the decrease in the ratio wIL/wCellulose does not seem to present a clear trend. Indeed, in entries 8, 10, and 9, the wIL/wCellulose ratios are 11.8, 10.8, and 6.8, respectively, while the conversions to levulinic acid are 22.7, 25.0, and 11.3% respectively. It can be seen that between entries 8 and 10 there is an increase in the conversion but, then, it decreases sharply. The effect of the wWater/wCellulose ratio on the conversion was the same. In entries 8, 10, and 9, the wWater/wCellulose ratios are 81.8, 75.0, and 34.0, respectively. The explanation for this negative result must be sought in the design of the experiment. Indeed, the experiment in entry 9 was carried out with a mass of sludge of almost 20 g, while in experiments 8 and 10 this mass was between 8 and 9 g. While the amount of cellulose available is higher, the amount of impurities even more. This must have disturbed the cellulose conversion reaction and maybe the mixing into the reactor. On the other hand, observing the mass balances of these experiments it can be seen that the balance for entry 9 was only 75%, while the mass balances for entries 8 and 10 were 90 and 98%, respectively. This fact could explain the bad result obtained with the experiment in entry 9.
The experiments realized with industrial and municipal sludge as received, entries 11 and 12 respectively, gave even smaller conversions to levulinic acid: 0.2 and 2.0%, respectively. The cause of this great decrease can be found in the fact that both sludges contain large amounts of ashes, 23.5 and 50.5%, respectively.
As it can be seen in
Table 5, levulinic acid represents more of the 90% of the total products. The other products are lactic acid, formic acid, HMF, and furfural. These other products were also found elsewhere [
3]. In
Figure 4, two chromatograms from HPLC analysis of products obtained from hydrothermal processing of cellulose are presented from industrial sludge (a) and municipal sludge (b).
In both chromatograms it is clearly showed that the higher peak (retention time 17.1 min) corresponds to levulinic acid. The other small peaks are the co-products identified in laboratory: furfural, lactic acid, formic acid, and 5-hydroxymethylfurfural (5-HMF). Further analysis of the products by LC–MS allowed to identify more substances in the products.
Table 6 presents the list of substances detected by LC–MS, the retention time of each compound and molecular mass of positive and negative ion electrospray analysis.
All compounds, except acetic acid, were detected in the products obtained from both types of sludge. Acetic acid was only presented in products obtained from municipal sludge. Almost all compounds are short-chain organic acid. These are the expected products of hydrolysis of the cellulose [
30]. Apart from organic acids, products of conversion of both sludges contain also glucose, furfural, and 5-HMF.
The peak of furfural has appeared in the chromatogram of conversion products of industrial sludge,
Figure 4a, but not in that of municipal sludge,
Figure 4b. LC–MS analysis confirmed the peak of furfural in both cases. Furfural comes from the dehydration of xylose. On the other hand, xylose makes up most of the hemicellulose. In
Table 4, it could be observed that the hemicellulose content in industrial sludge is double that in municipal sludge. For this reason, the furfural peak is observed in the chromatogram of industrial sludge and not in that of municipal sludge. In the case of 5-HMF, the conversion reactions were carried out at temperatures below 200 °C and therefore large amounts of 5-HMF were not produced. A 5-HMF peak cannot be seen in the chromatogram of conversion products of industrial sludge,
Figure 4a. However, in the chromatogram of conversion products of the municipal sludge,
Figure 4b, a very small one appears, as a result of which the 5-HMF was not completely dehydrated to levulinic acid.
On the other hand,
Table 5 also presents the weights and the yields of residual solids. Calculation was made based on the weight of biomass used in each reaction. The residual solids were in water insoluble dark-brown solids. The solids after filtration were abundantly washed with water to eliminate the remaining ionic liquid. Then, they were dried and weighted. As it can be seen in the table, depending on the source of cellulose used for the reaction of conversion to levulinic acid, the obtained yield of humins differ. Commercial cellulose (Sigmacell) obtained conversions to humins of ~20% except in the case of the experiment carried out for two hours, where the conversion reached was 66%. This increment was caused by insufficient the time to convert cellulose to products. In the case of all the other experiments, purified celluloses from municipal and industrial sludges, or from both sludges used as received, the conversions to humins were higher and quite similar, between 80 and 90%. The yields of residual wastes formed during the reactions with cellulose from sludges was due to the content of ash and proteins in municipal and industrial sludge. After cellulose extraction and purification, a part of ashes and proteins still remained in the cellulose. Subsequently, during the reaction, those organic and inorganic matters pass to the solid residue, at the same time, increasing the yield of humins.
Figure 5 shows the FTIR spectra of humins obtained after reaction of purified cellulose from both sludges.
In both spectra, it can be observed some characteristic absorbances in different frequency regions. The peak at 3400 cm
−1 is associated with hydroxyl group stretching vibrational bands. The peaks at 2950 cm
−1 and 1000–1250 cm
−1 are attributed to –CH and –CO stretching vibrations, respectively. It is also possible to observe strong peaks at 1700 and 1625 cm
−1, corresponding to carbonyl group conjugated to an alkene group. This is because carbohydrates are converted into humins according to the reaction pathway: cellulose → 5-HMF → 2,5-dioxo-6-hydroxyhexanal → humins [
14,
31]. Although, the peak at 1625 cm
−1 is also associated with peptide amide groups of proteins. The FTIR spectra confirm that except humins, ashes, proteins and part of unreacted cellulose goes to insoluble solids.
To summarize, the full process of production (extraction, bleaching, and catalyzed hydrothermal liquefaction) of levulinic acid from municipal or paper industry has been a success. It is true that the conversions obtained are not high, but it must be taken into account that the entire study is in fact a technological feasibility study. A scale-up of the process can only be carried out when some aspects of the process units will have been solved: (i) optimizing the extraction of cellulose from both sludges through an improved design of the ionic liquid; (ii) improve the cleaning of the cellulose after its extraction, although if more severe methods are used, a greater part of it can be hydrolyzed; and (iii) optimize the reaction operations in the conversion of cellulose to levulinic acid, reaction time and temperature, cellulose/ionic liquid/water ratios, or improvement in the design of the ionic liquid.