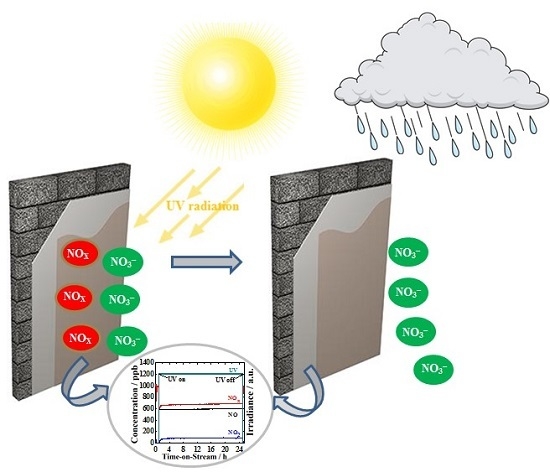
Photocatalytic Oxidation of NO over Composites of Titanium Dioxide and Zeolite ZSM-5
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Composite Photocatalysts
2.2. Photocatalytic Activity
2.3. Effect of Calcination Temperature
3. Experimental Section
3.1. Chemicals
3.2. Preparation of the TiO2/ZSM-5 Composites
3.2.1. Solid-State Dispersion
3.2.2. Sol-Gel Synthesis
3.2.3. Mechanical Mixture
3.3. Characterization of Photocatalysts
3.4. Photocatalytic Experiments
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farrell, A. Multi-lateral emission trading: Lessons from inter-state NOx control in the United States. Energy Policy 2001, 29, 1061–1072. [Google Scholar] [CrossRef]
- Castro, T.; Madronich, S.; Rivale, S.; Muhlia, A.; Mar, B. The influence of aerosols on photochemical smog in Mexico City. Atmos. Environ. 2001, 35, 1765–1772. [Google Scholar] [CrossRef]
- Devahasdin, S.; Fan, C.; Li, K.Y.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Cooper, C.D.; Alley, F.C. Air Pollution Control: A Design Approach, 4th ed.; Waveland Press: Long Grove, IL, USA, 2011; pp. 535–543. [Google Scholar]
- Tang, X.L.; Hao, J.M.; Xu, W.G.; Li, J.H. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2007, 8, 329–334. [Google Scholar] [CrossRef]
- Lim, T.H.; Jeong, S.M.; Kim, S.D.; Gyenis, J. Photocatalytic decomposition of NO by TiO2 particles. J. Photochem. Photobiol. A 2000, 134, 209–217. [Google Scholar] [CrossRef]
- Pleskov, Y.V.; Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 photocatalysis: Fundamentals and applications. Russ. J. Electrochem. 1999, 35, 1137–1138. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ibusuki, T.; Takeuchi, K. Removal of Low concentration nitrogen-oxides through photoassisted heterogeneous catalysis. J. Mol. Catal. 1994, 88, 93–102. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. TiO2 photocatalysts and diamond electrodes. Electrochim. Acta 2000, 45, 4683–4690. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. C. R. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Zhao, W.; Guan, B. Photocatalytic oxidation of nitrogen oxides using TiO2 loading on woven glass fabric. Chemosphere 2007, 66, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.S.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.G.; Liakou, M.; Gobin, C. Photocatalytic degradation of NOx gases using TiO2-containing paint: A real scale study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2436–2474. [Google Scholar] [CrossRef]
- Hassan, M.M.; Dylla, H.; Mohammad, L.N.; Rupnow, T. Evaluation of the durability of titanium dioxide photocatalyst coating for concrete pavement. Constr. Build. Mater. 2010, 24, 1456–1461. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Ringot, E.; Escadeillas, G. Degradation of NO using photocatalytic coatings to different substrates. Build. Environ. 2011, 46, 1808–1816. [Google Scholar] [CrossRef]
- Guo, S.; Wu, Z.; Zhao, W. TiO2-based building materials above and beyond traditional applications. Chin. Sci. Bull. 2009, 54, 1137–1142. [Google Scholar] [CrossRef]
- Hashimoto, K.; Wasada, K.; Toukai, N.; Kominami, H.; Kera, Y. Photocatalytic oxidation of nitrogen monoxide over titanium (IV) oxide nanocrystals large size areas. J. Photochem. Photobiol. A 2000, 136, 103–109. [Google Scholar] [CrossRef]
- Nakamura, I.; Sugihara, S.; Takeuchi, K. Mechanism for NO photooxidation over the oxygen-deficient TiO2 powder under visible light irradiation. Chem. Lett. 2000, 11, 1276–1277. [Google Scholar] [CrossRef]
- Dalton, J.S.; Janes, P.A.; Jones, N.G.; Nicholson, J.A.; Hallam, K.R.; Allen, G.C. Photocatalytic oxidation of NOx gases using TiO2: A surface spectroscopic approach. Environ. Pollut. 2002, 120, 415–422. [Google Scholar] [CrossRef]
- Ichiura, H.; Kitaoka, T.; Tanaka, H. Photocatalytic oxidation of NOx using composite sheets containing TiO2 and a metal compound. Chemosphere 2003, 51, 855–860. [Google Scholar] [CrossRef]
- Takeda, N.; Torimoto, T.; Sampath, S.; Kuwabata, S.; Yoneyama, H. Effect of inert supports for titanium-dioxide loading on enhancement of photodecomposition rate of gaseous propionaldehyde. J. Phys. Chem. 1995, 99, 9986–9991. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. B 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J.; Herrmann, J.M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Appl. Catal. B 1998, 18, 281–291. [Google Scholar] [CrossRef]
- Fichtner, S.; Hofmann, J.; Möller, A.; Schrage, C.; Giebelhausen, J.M.; Böhringer, B.; Gläser, R. Decomposition of 2-chloroethylethylsulfide on copper oxides to detoxify polymer-based spherical activated carbons from chemical warfare agents. J. Hazard. Mater. 2013, 262, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Tessinari, R.F.; Suarez, S.; Rasmussen, S.B.; Alonso, M.D.H.; Canela, M.C.; Avila, P.; Sanchez, B. Photocatalysis for continuous air purification in wastewater treatment plants: From lab to reality. Environ. Sci. Technol. 2012, 46, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, M.; Priya, S.V.; Arabindoo, B.; Palanicharnly, M.; Murugesan, V. Photocatalytic degradation of aqueous propoxur solution using TiO2 and H beta zeolite-supported TiO2. J. Hazard. Mater. 2009, 161, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Minagawa, M.; Matsuoka, M.; Yamashita, H.; Anpo, M. Photocatalytic decomposition of NO on Ti-HMS mesoporous zeolite catalysts. Catal. Lett. 2000, 66, 241–243. [Google Scholar] [CrossRef]
- Guo, G.F.; Hu, Y.; Jiang, S.M.; Wei, C.H. Photocatalytic oxidation of NOx over TiO2/HZSM-5 catalysts in the presence of water vapor: Effect of hydrophobicity of zeolites. J. Hazard. Mater. 2012, 223, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Wasada, K.; Osaki, M.; Shono, E.; Adachi, K.; Toukai, N.; Kominami, H.; Kera, Y. Photocatalytic oxidation of nitrogen oxide over titania-zeolite composite catalyst to remove nitrogen oxides in the atmosphere. Appl. Catal. B 2001, 30, 429–436. [Google Scholar] [CrossRef]
- Ichiura, H.; Kitaoka, T.; Tanaka, H. Preparation of composite TiO2-zeolite sheets using a papermaking technique and their application to environmental improvement—Part I—Removal of acetaldehyde with and without UV irradiation. J. Mater. Sci. 2002, 37, 2937–2941. [Google Scholar] [CrossRef]
- Ichiura, H.; Kitaoka, T.; Tanaka, H. Removal of indoor pollutants under UV irradiation by a composite TiO2-zeolite sheet prepared using a papermaking technique. Chemosphere 2003, 50, 79–83. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kimura, T.; Hidaka, M.; Rakhmawaty, D.; Anpo, M. Photocatalytic oxidation of acetaldehyde with oxygen on TiO2/ZSM-5 photocatalysts: Effect of hydrophobicity of zeolites. J. Catal. 2007, 246, 235–240. [Google Scholar] [CrossRef]
- Jansson, I.; Suárez, S.; Javier Garcia-Garcia, F.; Sánchez, B. Zeolite-TiO2 hybrid composites for pollutant degradation in gas phase. Appl. Catal. B 2015, 178, 100–107. [Google Scholar] [CrossRef]
- ISO. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semi Conducting Photocatalytic Materials—Part 1: Removal of Nitric oxide, 1st ed.; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Hu, Y.; Tsai, H.L.; Huang, C.L. Phase transformation of precipitated TiO2 nanoparticles. Mater. Sci. Eng. A 2003, 344, 209–214. [Google Scholar] [CrossRef]
- Wongkalasin, P.; Chavadej, S.; Sreethawong, T. Photocatalytic degradation of mixed azo dyes in aqueous wastewater using mesoporous-assembled TiO2 nanocrystal synthesized by a modified sol-gel process. Colloids Surf. A 2011, 384, 519–528. [Google Scholar] [CrossRef]
- Paola, A.D.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Rodella, C.B.; Nascente, P.A.P.; Franco, R.W.A.; Magon, C.J.; Mastelaro, V.R.; Florentino, A.O. Surface characterization of V2O5/TiO2 catalytic system. Phys. Status Solidi. A 2001, 187, 161–169. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.V.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Sang, S.Y.; Chang, F.X.; Liu, Z.M.; He, C.Q.; He, Y.L.; Xu, L. Difference of ZSM-5 zeolites synthesized with various templates. Catal. Today 2004, 93, 729–734. [Google Scholar] [CrossRef]
- Xu, Y.M.; Langford, C.H. Photoactivity of titanium dioxide supported on MCM41, zeolite X, and zeolite Y. J. Phys. Chem. B 1997, 101, 3115–3121. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kamegawa, T.; Ishiguro, Y.; Kido, R.; Yamashita, H. Design of composite photocatalyst of TiO2 and Y-zeolite for degradation of 2-propanol in the gas phase under UV and visible light irradiation. Molecules 2014, 19, 16477–16488. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. Mater. J. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Xu, Y.M.; Langford, C.H. Enhanced photoactivity of a titanium (IV) oxide supported on ZSM5 and zeolite A at low coverage. J. Phys. Chem. 1995, 99, 11501–11507. [Google Scholar] [CrossRef]
- Ma, J.; Wu, H.; Liu, Y.; He, H. Photocatalytic removal of NOx over visible light responsive oxygen-deficient TiO2. J. Phys. Chem. C 2014, 118, 7434–7441. [Google Scholar] [CrossRef]







| Catalysts | µmol·m−2·h−1 | µmol ·m−2·h−1 | µmol·m−2·h−1 | |||
|---|---|---|---|---|---|---|
| P25 | 45 | 16 | 704 | 455 | 249 | 65 |
| TiO2 | 32 | 19 | 601 | 291 | 310 | 48 |
| Hombikat | 35 | 16 | 518 | 271 | 247 | 52 |
| TSSSG | 31 | 12 | 502 | 305 | 197 | 62 |
| HZMM (25/75) | 16 | 9 | 257 | 108 | 149 | 33 |
| HZMM (50/50) | 21 | 14 | 327 | 103 | 224 | 31 |
| HZMM (75/25) | 24 | 16 | 373 | 132 | 241 | 38 |
| HZSSD (25/75) | 28 | 17 | 435 | 163 | 271 | 37 |
| HZSSD (50/50) | 30 | 22 | 553 | 169 | 384 | 30 |
| HZSSD (75/25) | 29 | 19 | 450 | 160 | 290 | 35 |
| TZSG (25/75) | 31 | 23 | 483 | 123 | 360 | 25 |
| TZSG (50/50) | 41 | 33 | 655 | 131 | 524 | 19 |
| TZSG (75/25) | 36 | 27 | 572 | 166 | 406 | 29 |
| Samples | NO3−/mg·g−1 |
|---|---|
| Hombikat | 0.30 |
| P25 | 0.26 |
| TSSSG | 0.22 |
| TZSG (50/50) | 0.66 |
| Tcalcination/K | Crystallite Size/nm | ABET/(m2·g−1) | dBJH/nm |
|---|---|---|---|
| 423 | 6 | 290 | 4.16 |
| 523 | 8 | 278 | 4.49 |
| 623 | 8 | 265 | 4.57 |
| 723 | 9 | 219 | 5.80 |
| 823 | 23 | 193 | 7.62 |
| Tcalcinaton/K | µmol·m−2·h−1 | µmol·m−2·h−1 | µmol·m−2·h−1 | |||
|---|---|---|---|---|---|---|
| 423 | 30 | 19 | 494 | 287 | 280 | 48 |
| 523 | 41 | 33 | 655 | 131 | 524 | 19 |
| 623 | 38 | 30 | 608 | 119 | 447 | 19 |
| 723 | 35 | 28 | 548 | 109 | 400 | 20 |
| 823 | 27 | 21 | 429 | 150 | 292 | 25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawari, A.; Einicke, W.-D.; Gläser, R. Photocatalytic Oxidation of NO over Composites of Titanium Dioxide and Zeolite ZSM-5. Catalysts 2016, 6, 31. https://doi.org/10.3390/catal6020031
Tawari A, Einicke W-D, Gläser R. Photocatalytic Oxidation of NO over Composites of Titanium Dioxide and Zeolite ZSM-5. Catalysts. 2016; 6(2):31. https://doi.org/10.3390/catal6020031
Chicago/Turabian StyleTawari, Akram, Wolf-Dietrich Einicke, and Roger Gläser. 2016. "Photocatalytic Oxidation of NO over Composites of Titanium Dioxide and Zeolite ZSM-5" Catalysts 6, no. 2: 31. https://doi.org/10.3390/catal6020031