A Critical Review of Recent Progress and Perspective in Practical Denitration Application
Abstract
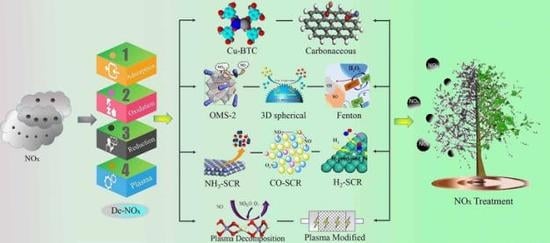
:1. Introduction
2. Removal of NOx by Adsorption
2.1. NOx Removed by Cu-BTC Adsorption
2.2. NOx Removed by Carbonaceous Material Adsorption
2.3. Other Crucial NOx Adsorbents
3. Removal of NOx by Oxidation
3.1. NOx Removed by Gas-Phase oxidation
3.2. NOx Removed by Liquid-Phase Oxidation
3.3. NOx Removed by Catalytic Oxidation
3.3.1. Catalytic Oxidation of Molecular Sieve
3.3.2. Catalytic Oxidation of Metal Oxides
3.3.3. Photocatalytic Oxidation
3.3.4. Catalytic Oxidation of Fenton System
4. Removal of NOx by Reduction
4.1. Selective Noncatalytic Reduction (SNCR) for NOx Removal
4.2. Selective Catalytic Reduction (SCR) for NOx Removal
4.2.1. NH3-SCR
- (i)
- High-temperature De-NOx unit at high dust content. In this unit, the temperature of the flue gas entering the NH3-SCR reactor is as high as 300–400 °C. This high temperature significantly favors the De-NOx performance of most of the catalysts (e.g., vanadium-type-based catalysts) [150,151]. At present, this technology is the most widely used in SIIs. However, further progress in promising applications is still somewhat bottlenecked by (a) the high dust content in the flue gas, which easily leads to erosion and plugging holes in MHCs and (b) the high SO2 content of the flue gas, leading to catalyst poisoning and deactivation. These limitations are yet to be overcome [152].
- (ii)
- High-temperature De-NOx unit at high SO2 content. In this unit, the temperature of the flue gas entering the NH3-SCR reactor ranges from 180 to 280 °C. A dust-removing apparatus is used to mitigate the erosion and plugging issues for longer service life of MHCs for NH3-SCR. Nevertheless, the high SO2 content continues to raise greater worries for activity declining and shorter service life [153,154]. Most of manganese- and copper-based catalysts with high SO2 resistance could be used in this process [155,156].
- (iii)
- Low-temperature De-NOx unit at high H2O content. In this unit, the temperature of the flue gas entering the NH3-SCR reactor is below 160 °C. Although dust and SO2 are previously removed, the H2O content in the flue gas still remains as high as 10 vol.%. Almost all current catalysts show low activity and poor water-resistance at this temperature [157,158]. Therefore, this technique is not practical for SIIs.
4.2.2. CO-SCR
4.2.3. HC-SCR
4.2.4. H2-SCR
5. Removal of NOx by Plasma
5.1. Direct Decomposition of NOx by Plasma
5.2. Plasma-Modified Catalyst
5.3. Plasma Concerted Catalysis
6. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Seitzinger, S.P.; Phillips, L. Nitrogen stewardship in the Anthropocene. Science 2017, 357, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Tvw, J.; Pnr, V. A molecular dance to cleaner air. Science 2017, 357, 866–867. [Google Scholar]
- Boyle, E. Nitrogen pollution knows no bounds. Science 2017, 356, 700. [Google Scholar] [CrossRef] [PubMed]
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Xu, Z.; Wu, G.C.; Mao, Y.M.; Liu, L.N.; Qian, W.; Dan, Y.L.; Tao, S.S.; Zhang, Q.; Sam, N.B.; et al. Emerging role of air pollution in autoimmune diseases. Autoimmun. Rev. 2019, 18, 507–614. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest 2019, 155, 417–426. [Google Scholar] [CrossRef]
- Guo, T.; Wang, Y.; Zhang, H.; Zhang, Y.; Zhao, J.; Wang, Q.; Shen, H.; Wang, Y.; Xie, X.; Wang, L.; et al. The association between ambient PM2.5 exposure and the risk of preterm birth in China: A retrospective cohort study. Sci. Total Environ. 2018, 633, 1453–1459. [Google Scholar] [CrossRef]
- Zeng, Q.; Darboux, F.; Man, C.; Zhu, Z.; An, S. Soil aggregate stability under different rain conditions for three vegetation types on the Loess Plateau (China). CATENA 2018, 167, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Du, E.; Dong, D.; Zeng, X.; Sun, Z.; Jiang, X.; de Vries, W. Direct effect of acid rain on leaf chlorophyll content of terrestrial plants in China. Sci. Total Environ. 2017, 605, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.Q.; Nguyen, H.D.; Vu, K.; Hien, T.T. Photochemical Smog Modelling Using the Air Pollution Chemical Transport Model (TAPM-CTM) in Ho Chi Minh City, Vietnam. Environ. Model. Assess. 2019, 24, 295–310. [Google Scholar] [CrossRef]
- Lewis, A.C. The changing face of urban air pollution. Science 2018, 359, 744. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, W.; Sardans, J.; An, W.; Zeng, C.; Abid, A.A.; Peñuelas, J. Effect of simulated acid rain on CO2, CH4 and N2O fluxes and rice productivity in a subtropical Chinese paddy field. Environ. Pollut. 2018, 243, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guo, S.; Zhang, M. Assessing customers’ perceived value of the anti-haze cosmetics under haze pollution. Sci. Total Environ. 2019, 685, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Chong, X.; Wang, Y.; Liu, R.; Zhang, Y.; Zhang, Y.; Zheng, W. Pollution characteristics and source difference of gaseous elemental mercury between haze and non-haze days in winter. Sci. Total Environ. 2019, 678, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Jia, B.; Roskilly, A.P. Application of Miller cycle with turbocharger and ethanol to reduce NOx and particulates emissions from diesel engine—A numerical approach with model validations. Appl. Therm. Eng. 2019, 150, 904–911. [Google Scholar] [CrossRef]
- Gu, Y.; Epling, W.S. Passive NOx adsorber: An overview of catalyst performance and reaction chemistry. Appl. Catal. Gen. 2019, 570, 1–14. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, S.; Ding, J.; Meng, Y.; Zhong, Q.; Kong, D.; Gu, C. Effect of adsorption properties of phosphorus-doped TiO2 nanotubes on photocatalytic NO removal. J. Colloid Interface Sci. 2019, 553, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Okoshi, M.; Ishikawa, A.; Nakai, H. Temperature- and pressure-dependent adsorption configuration of NO molecules on Rh(111) surface: A theoretical study. Surf. Sci. 2019, 686, 58–62. [Google Scholar] [CrossRef]
- Wang, T.; Ding, L.; Song, Y.; Cuiqing, L.I.; Wang, H.; Ren, X. Performance of simultaneous denitrification and desulfurization for Y2O3/AC catalysts. Chin. J. Environ. Eng. 2016, 4, 132–139. [Google Scholar]
- Liu, Y.; Zhang, X.; Ding, J. Chemical effect of NO on CH4 oxidation during combustion in O2/NO environments. Chem. Phys. Lett. 2019, 727, 59–65. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Yang, Z.; Cao, F.; Li, L.; Chen, H.; Liu, H.; Xiong, K.; Wu, J.; Hong, Z.; et al. Electrospun YMn2O5 nanofibers: A highly catalytic activity for NO oxidation. Appl. Catal. Environ. 2019, 247, 133–141. [Google Scholar] [CrossRef]
- Jo, D.; Park, G.T.; Ryu, T.; Hong, S.B. Economical synthesis of high-silica LTA zeolites: A step forward in developing a new commercial NH3-SCR catalyst. Appl. Catal. Environ. 2019, 243, 212–219. [Google Scholar] [CrossRef]
- Chitpakdee, C.; Junkaew, A.; Maitarad, P.; Shi, L.; Promarak, V.; Kungwan, N.; Namuangruk, S. Understanding the role of Ru dopant on selective catalytic reduction of NO with NH3 over Ru-doped CeO2 catalyst. Chem. Eng. J. 2019, 369, 124–133. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Liu, X.; Ma, C.; Ding, X.; Yan, W. Directly catalytic reduction of NO without NH3 by single atom iron catalyst: A DFT calculation. Fuel 2019, 243, 262–270. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, J.; Wang, B.; Lou, J.; Li, Y.; Li, X.; Li, Z.; Liu, X.; Meng, Q.; Gao, P.; et al. Plasma-catalytic high-efficiency oxidation of NO over Co-Mn/Ti catalysts using surface dielectric barrier discharge plasma. Vacuum 2019, 167, 249–254. [Google Scholar] [CrossRef]
- Wang, J.; Yi, H.; Tang, X.; Zhao, S.; Gao, F. Exceptional adsorptive capacity and kinetic of γ-Al2O3 for selective adsorption of NO assisted by nonthermal plasma. Chem. Eng. J. 2019, 378, 122095. [Google Scholar] [CrossRef]
- Xie, S.; Ren, W.; Qiao, C.; Tong, K.; Sun, J.; Zhang, M.; Liu, X.; Zhang, Z. An electrochemical adsorption method for the reuse of waste water-based drilling fluids. Nat. Gas Ind. B 2018, 5, 508–512. [Google Scholar] [CrossRef]
- Ikeda, H.; Koike, Y.; Shiratori, K.; Ueda, K.; Shirahata, N.; Isegawa, K.; Toyoshima, R.; Masuda, S.; Mase, K.; Nito, T.; et al. Adsorption state of NO on Ir (111) surfaces under excess O2 coexisting condition. Surf. Sci. 2019, 685, 1–6. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Wang, T. Adsorption of NO from flue gas by molecularly imprinted adsorbents. Chem. Eng. J. 2016, 306, 832–839. [Google Scholar] [CrossRef]
- Hastürk, E.; Ernst, S.J.; Janiak, C. Recent advances in adsorption heat transformation focusing on the development of adsorbent materials. Curr. Opin. Chem. Eng. 2019, 24, 26–36. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, T.; Yin, G.; Xu, S.; Zhang, Q.; Su, H. Effect of properties of activated carbon on malachite green adsorption. Fuel 2019, 249, 45–53. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Ultrahigh benzene adsorption capacity of graphene-MOF composite fabricated via MOF crystallization in 3D mesoporous graphene. Microporous Mesoporous Mater. 2019, 279, 387–394. [Google Scholar] [CrossRef]
- Sherino, B.; Halim, S.N.A.; Manan, N.S.A.; Sarip, R.; Al’Abri, A.M.; Mohamad, S. Facile synthesis and characterization of novel dicarboxylate-Cu based MOFs materials. Inorg. Chim. Acta 2019, 491, 59–66. [Google Scholar] [CrossRef]
- Qin, Y.H.; Huang, L.; Zhang, L.; He, H. One-step synthesis of confined ion Agx-Cu-BTC for selective catalytic reduction of NO with CO. Inorg. Chem. Commun. 2019, 102, 130–133. [Google Scholar] [CrossRef]
- Khan, A.H.; Peikert, K.; Fröba, M.; Bertmer, M. NO adsorption in amino-modified Cu3(btc)2-type MOFs studied by solid-state NMR. Microporous Mesoporous Mater. 2015, 216, 111–117. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Metal organic framework (MOF) porous octahedral nanocrystals of Cu-BTC: Synthesis, properties and enhanced adsorption properties. Mater. Res. Bull. 2019, 109, 124–133. [Google Scholar] [CrossRef]
- Meng, G.; Song, X.; Ji, M.; Hao, J.; Shi, Y.; Ren, S.; Qiu, J.; Hao, C. Molecular simulation of adsorption of NO and CO2 mixtures by a Cu-BTC metal organic framework. Curr. Appl. Phys. 2015, 15, 1070–1074. [Google Scholar] [CrossRef]
- Qin, Y.H.; Huang, L.; Zhang, D.L.; Sun, L.G. Mixed-node A-Cu-BTC and porous carbon based oxides derived from A-Cu-BTC as low temperature NO–CO catalyst. Inorg. Chem. Commun. 2016, 66, 64–68. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.; Ying, C.; Fei, X.; Li, W.; Li, Y.; Chen, M. Adsorption properties of activated carbon from reed with a high adsorption capacity. Ecol. Eng. 2017, 102, 443–450. [Google Scholar]
- Vanraes, P.; Ghodbane, H.; Davister, D.; Wardenier, N.; Nikiforov, A.; Verheust, Y.P.; Swh, V.H.; Hamdaoui, O.; Vandamme, J.; Van, D.J. Removal of several pesticides in a falling water film DBD reactor with activated carbon textile: Energy efficiency. Water Res. 2017, 116, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beijer, K.; Björlenius, B.; Shaik, S.; Lindberg, R.H.; Brunström, B.; Brandt, I. Removal of pharmaceuticals and unspecified contaminants in sewage treatment effluents by activated carbon filtration and ozonation: Evaluation using biomarker responses and chemical analysis. Chemosphere 2017, 176, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Kou, L.; Wang, T.; Liang, D.; Hu, S. Evaluation of activated carbon fiber supported nanoscale zero-valent iron for chromium (VI) removal from groundwater in a permeable reactive column. J. Environ. Manag. 2017, 201, 378. [Google Scholar] [CrossRef] [PubMed]
- Shimabuku, K.K.; Paige, J.M.; Lunaaguero, M.; Summers, R.S. Simplified Modeling of Organic Contaminant Adsorption by Activated Carbon and Biochar in the Presence of Dissolved Organic Matter and Other Competing Adsorbates. Environ. Sci. Technol. 2017, 51, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, Y.; Hui, W.; Zhao, S.; Chen, M.; Meng, L.; Wei, H. Effects of Acidic Gases on Mercury Adsorption by Activated Carbon in Simulated Oxy-Fuel Combustion Flue Gas. Energy Fuels 2017, 31, 9745–9751. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Munusamy, K.; Somani, R.S.; John, M.; Newalkar, B.L.; Bajaj, H.C. Precursor suitability and pilot scale production of super activated carbon for greenhouse gas adsorption and fuel gas storage. Chem. Eng. J. 2017, 315, 415–425. [Google Scholar] [CrossRef]
- Fałtynowicz, H.; Hodurek, P.; Kaczmarczyk, J.; Kułażyński, M.; Łukaszewicz, M. Hydrolysis of surfactin over activated carbon. Bioorg. Chem. 2019, 13, 145–149. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Mohammadzadeh, A.; Tran, H.N.; Anastopoulos, I.; Dotto, G.L.; Lopičić, Z.R.; Sivamani, S.; Rahmani-Sani, A.; Ivanets, A.; Hosseini-Bandegharaei, A. Efficient mercury removal from wastewater by pistachio wood wastes-derived activated carbon prepared by chemical activation using a novel activating agent. J. Environ. Manag. 2018, 223, 1001–1009. [Google Scholar] [CrossRef]
- Ouhammou, M.; Lahnine, L.; Mghazli, S.; Hidar, N.; Bouchdoug, M.; Jaouad, A.; Mandi, L.; Mahrouz, M. Valorisation of cellulosic waste basic cactus to prepare activated carbon. J. Saudi Soc. Agric. Sci. 2019, 18, 133–140. [Google Scholar] [CrossRef]
- Björklund, K.; Li, L.Y. Adsorption of organic stormwater pollutants onto activated carbon from sewage sludge. J. Environ. Manag. 2017, 197, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benstoem, F.; Becker, G.; Firk, J.; Kaless, M.; Wuest, D.; Pinnekamp, J.; Kruse, A. Elimination of micropollutants by activated carbon produced from fibers taken from wastewater screenings using hydrothermal carbonization. J. Environ. Manag. 2018, 211, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Pirsaheb, M.; Mohamadi, S.; Rahmatabadi, S.; Hossini, H.; Motteran, F. Simultaneous wastewater treatment and biogas production using integrated anaerobic baffled reactor granular activated carbon from baker’s yeast wastewater. Environ. Technol. 2018, 39, 2724–2735. [Google Scholar] [CrossRef] [PubMed]
- Jahandar, L.M.; Atkinson, J.D.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. The role of beaded activated carbon’s surface oxygen groups on irreversible adsorption of organic vapors. J. Hazard. Mater. 2016, 317, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.; Li, G.; Zhang, J.; Guo, Y. NO removal activity and surface characterization of activated carbon with oxidation modification. J. Energy Inst. 2017, 90, 813–823. [Google Scholar] [CrossRef]
- Ren, S.; Guo, F.; Yang, J.; Yao, L.; Zhao, Q.; Kong, M. Selection of carbon materials and modification methods in low-temperature sintering flue gas denitrification. Chem. Eng. Res. Des. 2017, 126, 278–285. [Google Scholar] [CrossRef]
- Jie, W.; Lin, H.; Xue, H.; Ying, Z.; Wei, C. Physicochemical studies of adsorptive denitrogenation by oxidized activated carbons. Ind. Eng. Chem. Res. 2017, 56, 5033–5041. [Google Scholar]
- Chong-jiu, L.; Zhao, R.; Meng-qi, P.; Liu, H.; Gang, Y.U.; Xia, D.S. Study on desulfurization and denitrification by modified activated carbon fibers with visible-light photocatalysis. J. Fuel Chem. Technol. 2015, 43, 1516–1522. [Google Scholar]
- Arcibar-Orozco, J.A.; Acosta-Herrera, A.A.; Rangel-Mendez, J.R. Simultaneous desulfuration and denitrogenation of model diesel fuel by Fe-Mn microwave modified activated carbon: Iron crystalline habit influence on adsorption capacity. J. Clean. Prod. 2019, 218, 69–82. [Google Scholar] [CrossRef]
- You, F.T.; Yu, G.W.; Xing, Z.J.; Li, J.; Xie, S.Y.; Li, C.X.; Wang, G.; Ren, H.Y.; Wang, Y. Enhancement of NO catalytic oxidation on activated carbon at room temperature by nitric acid hydrothermal treatment. Appl. Surf. Sci. 2019, 471, 633–644. [Google Scholar] [CrossRef]
- Sun, X.; Sun, L.; Liu, Y.; Li, K.; Wang, C.; Song, X.; Ning, P. Research on reaction conditions and mechanism for simultaneous removal of NO and Hg0 over CuFe modified activated carbon at low temperature. J. Energy Inst. 2019, 6, 223–228. [Google Scholar]
- Wei, Z.S.; Zeng, G.H.; Xie, Z.R.; Ma, C.Y.; Liu, X.H.; Sun, J.L.; Liu, L.H. Microwave catalytic NOx and SO2 removal using FeCu/zeolite as catalyst. Fuel 2011, 90, 1599–1603. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Sun, Q.; Xu, W.Q.; Ma, Z.; Wang, H.; Zhang, D.; Zhou, J. Novel synthesis of fly-ash-derived Cu-loaded SAPO-34 catalysts and their use in selective catalytic reduction of NO with NH3. Green Energy Environ. 2019, 32, 234–239. [Google Scholar] [CrossRef]
- Agote-Arán, M.; Lezcano-González, I.; Greenaway, A.G.; Hayama, S.; Díaz-Moreno, S.; Kroner, A.B.; Beale, A.M. Operando HERFD-XANES/XES studies reveal differences in the activity of Fe-species in MFI and CHA structures for the standard selective catalytic reduction of NO with NH3. Appl. Catal. A: Gen. 2019, 570, 283–291. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Cong, Q.; Ma, H.; Li, S.; Li, W. Design of a hierarchical Fe-ZSM-5@CeO2 catalyst and the enhanced performances for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2019, 369, 957–967. [Google Scholar] [CrossRef]
- Baran, R.; Averseng, F.; Wierzbicki, D.; Chalupka, K.; Krafft, J.-M.; Grzybek, T.; Dzwigaj, S. Effect of postsynthesis preparation procedure on the state of copper in CuBEA zeolites and its catalytic properties in SCR of NO with NH3. Appl. Catal. A: Gen. 2016, 523, 332–342. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Liu, E.; Wan, J.; Hu, X.; Fan, J. High efficiency for H2 evolution and NO removal over the Ag nanoparticles bridged g-C3N4 and WS2 heterojunction photocatalysts. Appl. Catal. B: Environ. 2017, 219, 467–478. [Google Scholar] [CrossRef]
- Imai, S.; Miura, H.; Shishido, T. Selective catalytic reduction of NO with CO and C3H6 over Rh/NbOPO4. Catal. Today 2019, 332, 267–271. [Google Scholar] [CrossRef]
- Xiao, P.; Davis, R.C.; Ouyang, X.; Li, J.; Thomas, A.; Scott, S.L.; Zhu, J. Mechanism of NO reduction by CO over Pt/SBA-15. Catal. Commun. 2014, 50, 69–72. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Wang, Z.; Ma, C.; Qin, Y. Investigation on Fe-Co binary metal oxides supported on activated semi-coke for NO reduction by CO. Appl. Catal. B: Environ. 2017, 201, 636–651. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Hu, F.; Qin, L.; Han, J.; Wu, G. In-situ DRIFTS investigation on the selective catalytic reduction of NO with NH3 over the sintered ore catalyst. Appl. Surf. Sci. 2018, 439, 75–81. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Wen, L.; Yang, C.; Zhang, L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere 2019, 224, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ning, P.; Zhang, Q.; Liu, X.; Zhang, T.; Fan, J.; Wang, J.; Long, K. Promotional mechanism of WO3 over RuO2-Fe2O3 catalyst for NH3-SCO reaction. Appl. Catal. A: Gen. 2018, 561, 158–167. [Google Scholar] [CrossRef]
- Ibusuki, T.; Takeuchi, K. Removal of low concentration nitrogen oxides through photoassisted heterogeneous catalysis. J. Mol. Catal. 1994, 88, 93–102. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H.; Liu, L.; Jiang, H. Revisit the effect of manganese oxidation state on activity in low-temperature NO-SCR. Mol. Catal. 2018, 446, 49–57. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Q.; Ran, G.; Kong, M.; Ren, S.; Yang, J.; Li, J. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem. Eng. J. 2019, 370, 810–821. [Google Scholar] [CrossRef]
- Lin, F.; Shao, J.; Tang, H.; Li, Y.; Wang, Z.; Chen, G.; Yuan, D.; Cen, K. Enhancement of NO oxidation activity and SO2 resistance over LaMnO3+δ perovskites catalysts with metal substitution and acid treatment. Appl. Surf. Sci. 2019, 479, 234–246. [Google Scholar] [CrossRef]
- Salman, A.U.R.; Enger, B.C.; Auvray, X.; Lødeng, R.; Menon, M.; Waller, D.; Rønning, M. Catalytic oxidation of NO to NO2 for nitric acid production over a Pt/Al2O3 catalyst. Appl. Catal. A: Gen. 2018, 564, 142–146. [Google Scholar] [CrossRef]
- Jia, J.; Ran, R.; Guo, X.; Wu, X.; Chen, W.; Weng, D. Enhanced low-temperature NO oxidation by iron-modified MnO2 catalysts. Catal. Commun. 2019, 119, 139–143. [Google Scholar] [CrossRef]
- Yuan, P.; Egedy, A.; Miskolczi, N.; Shen, B.; Wang, J.; Zhou, W.; Pan, Y.; Zhang, H. Oxidation removal of NO by in situ Fenton system: Factors and optimization. Fuel 2018, 233, 519–528. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, R.; Wang, T.; Yang, C. Follow-up research for integrative process of pre-oxidation and post-absorption cleaning flue gas: Absorption of NO2, NO and SO2. Chem. Eng. J. 2015, 273, 55–65. [Google Scholar] [CrossRef]
- Guo, L.; Han, C.; Zhang, S.; Zhong, Q.; Ding, J.; Zhang, B.; Zeng, Y. Enhancement effects of O2− and OH radicals on NOX removal in the presence of SO2 by using an O3/H2O2 AOP system with inadequate O3 (O3/NO molar ratio = 0.5). Fuel 2018, 233, 769–777. [Google Scholar] [CrossRef]
- Fei, L.; Lin, Q.; Bi, X.; Chan, L.Y.; Chen, S.; Yan, X.; Liu, Y.; Wang, X.; Zhang, Y.; Cen, C. Effect of lightning activities on surface atmospheric NO, O3 and submicron particles based on artificially triggered lightning technology: A case study. Atmos. Pollut. Res. 2019, 10, 105–110. [Google Scholar] [CrossRef]
- Hultén, A.H.; Nilsson, P.; Samuelsson, M.; Ajdari, S.; Normann, F.; Andersson, K. First evaluation of a multicomponent flue gas cleaning concept using chlorine dioxide gas—Experiments on chemistry and process performance. Fuel 2017, 210, 885–891. [Google Scholar] [CrossRef]
- Sun, W.Y.; Ding, S.L.; Zeng, S.S.; Su, S.J.; Jiang, W.J. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone. J. Hazard. Mater. 2011, 192, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Mok, Y.S.; LEE, H.J. Removal of sulfur dioxide and nitrogen oxides by using ozone injection and absorption-reduction technique. Fuel Process. Technol. 2006, 87, 591–597. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Ma, Q.; Yang, Y.; Whiddon, R.; Zhu, Y.; Cen, K. Catalytic deep oxidation of NO by ozone over MnOx loaded spherical alumina catalyst. Appl. Catal. B: Environ. 2016, 198, 100–111. [Google Scholar] [CrossRef]
- Han, C.; Zhang, S.; Guo, L.; Zeng, Y.; Li, X.; Shi, Z.; Zhang, Y.; Zhang, B.; Zhong, Q. Ehanced catalytic ozonation of NO over black-TiO2 catalyst under inadequate ozone (O3/NO molar ratio = 0.6). Chem. Eng. Res. Des. 2018, 136, 219–229. [Google Scholar] [CrossRef]
- Hao, R.; Mao, X.; Wang, Z.; Zhao, Y.; Wang, T.; Sun, Z.; Yuan, B.; Li, Y. A novel method of ultraviolet/NaClO2-NH4OH for NO removal: Mechanism and kinetics. J. Hazard. Mater. 2019, 368, 234–242. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, T.X.; Chen, Z.Y.; Du, Y.R. Simultaneous removal of SO2 and NO using M/NaClO2 complex absorbent. Chem. Eng. J. 2010, 160, 42–47. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Si, H.L.; Jung, J.H.; Shon, B.H.; Lee, H.K. Study on the removal of NO_x from simulated flue gas using acidic NaClO_2 solution. J. Environ. Sci. 2008, 20, 33–38. [Google Scholar] [CrossRef]
- Hao, R.; Yang, S.; Bo, Y.; Yi, Z. Simultaneous desulfurization and denitrification through an integrative process utilizing NaClO2/Na2S2O8. Fuel Process. Technol. 2017, 159, 145–152. [Google Scholar] [CrossRef]
- Yang, S.; Pan, X.; Han, Z.; Zheng, D.; Yu, J.; Xia, P.; Liu, B.; Yan, Z. Nitrogen Oxide Removal from Simulated Flue Gas by UV-Irradiated Sodium Chlorite Solution in a Bench-Scale Scrubbing Reactor. Ind. Eng. Chem. Res. 2017, 56, 3671–3678. [Google Scholar] [CrossRef]
- Wang, W.; Guo, R.; Pan, W.; Hu, G. Low temperature catalytic oxidation of NO over different-shaped CeO2. J. Rare Earths 2018, 36, 588–593. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Wang, Y.; Li, X. Hierarchically porous LaFeO3 perovskite prepared from the pomelo peel bio-template for catalytic oxidation of NO. J. Phys. Chem. Solids 2018, 116, 43–49. [Google Scholar] [CrossRef]
- Shang, H.; Li, Y.; Liu, J.; Tang, X.; Yang, J.; Li, J. CH4/N2 separation on methane molecules grade diameter channel molecular sieves with a CHA-type structure. Chin. J. Chem. Eng. 2018, 1, 102–110. [Google Scholar] [CrossRef]
- Yamane, Y.; Tanaka, H.; Miyahara, M.T. In silico synthesis of carbon molecular sieves for high-performance air separation. Carbon 2019, 141, 626–634. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Li, H.; Yang, J.; Yang, Z.; Zhao, J.; Peng, H.; Shih, K. Elemental mercury oxidation over manganese oxide octahedral molecular sieve catalyst at low flue gas temperature. Chem. Eng. J. 2019, 356, 142–150. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, L.J.; Yang, C.; Zhang, H.Y.; Zhao, Y.R.; Fan, H.L.; Huo, C. Room-temperature hydrogen sulfide removal with zinc oxide nanoparticle/molecular sieve prepared by melt infiltration. Fuel Process. Technol. 2019, 185, 26–37. [Google Scholar] [CrossRef]
- Dharmarathna, S.; King’ondu, C.K.; Pahalagedara, L.; Kuo, C.H.; Zhang, Y.; Suib, S.L. Manganese octahedral molecular sieve (OMS-2) catalysts for selective aerobic oxidation of thiols to disulfides. Appl. Catal. B: Environ. 2014, 147, 124–131. [Google Scholar] [CrossRef]
- Park, D.; Ju, Y.; Kim, J.H.; Ahn, H.; Lee, C.H. Equilibrium and kinetics of nitrous oxide, oxygen and nitrogen adsorption on activated carbon and carbon molecular sieve. Sep. Purif. Technol. 2019, 223, 63–80. [Google Scholar] [CrossRef] [Green Version]
- Sarmah, B.; Srivastava, R. Selective two-step synthesis of 2,5-diformylfuran from monosaccharide, disaccharide, and polysaccharide using H-Beta and octahedral MnO2 molecular sieves. Mol. Catal. 2019, 462, 92–103. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Tanaka, T.; Takahashi, N.; Tsukamoto, Y.; Takagi, N.; Shinjoh, H. Low-temperature NO-adsorption properties of manganese oxide octahedral molecular sieves with different potassium content. Appl. Catal. B: Environ. 2016, 193, 234–239. [Google Scholar] [CrossRef]
- Tolaymat, T.; El Badawy, A.; Genaidy, A.; Abdelraheem, W.; Sequeira, R. Analysis of metallic and metal oxide nanomaterial environmental emissions. J. Clean. Prod. 2017, 143, 401–412. [Google Scholar] [CrossRef]
- Jõgi, I.; Erme, K.; Raud, J.; Laan, M. Oxidation of NO by ozone in the presence of TiO2 catalyst. Fuel 2016, 173, 45–51. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wang, Y.; Lyu, Y.K. Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem. Eng. J. 2019, 361, 1161–1172. [Google Scholar] [CrossRef]
- Ma, S.J.; Wang, X.W.; Chen, T.; Yuan, Z.H. Effect of surface morphology on catalytic activity for NO oxidation of SmMn2O5 nanocrystals. Chem. Eng. J. 2018, 354, 191–196. [Google Scholar] [CrossRef]
- Cai, W.; Zhao, Y.; Chen, M.; Jiang, X.; Wang, H.; Ou, M.; Wan, S.; Zhong, Q. The formation of 3D spherical Cr-Ce mixed oxides with roughness surface and their enhanced low-temperature NO oxidation. Chem. Eng. J. 2017, 333, 414–422. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Ho, W.; Cao, J.; Shen, Z.; Lee, S.C. Fabrication of Bi2O2CO3/g-C3N4 heterojunctions for efficiently photocatalytic NO in air removal: In-situ self-sacrificial synthesis, characterizations and mechanistic study. Appl. Catal. B: Environ. 2016, 199, 123–133. [Google Scholar] [CrossRef]
- Ângelo, J.; Andrade, L.; Madeira, L.M.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Luo, J.; Zhou, S.; Mao, X.; Shah, M.W.; Wang, F.; Chen, Z.; Wang, C. TiO2-supported Ag nanoclusters with enhanced visible light activity for the photocatalytic removal of NO. Appl. Catal. B: Environ. 2018, 234, 206–212. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Wang, I.; Wu, J.; Yang, Y.; An, Q. Carbon wrapped and doped TiO2 mesoporous nanostructure with efficient visible-light photocatalysis for NO removal. Appl. Surf. Sci. 2017, 391, 318–325. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Li, H.; Li, Y.; Zhao, Y.; Zheng, C. Simultaneous removal of SO2, NO and mercury using TiO2-aluminum silicate fiber by photocatalysis. Chem. Eng. J. 2012, 192, 21–28. [Google Scholar] [CrossRef]
- Jin, S.; Dong, G.; Luo, J.; Ma, F.; Wang, C. Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst. Appl. Catal. B: Environ. 2018, 227, 24–34. [Google Scholar] [CrossRef]
- Huy, T.H.; Bui, D.P.; Kang, F.; Wang, Y.F.; Liu, S.H.; Thi, C.M.; You, S.J.; Chang, G.M.; Pham, V.V. SnO2/TiO2 nanotube heterojunction: The first investigation of NO degradation by visible light-driven photocatalysis. Chemosphere 2019, 215, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Li, X.; Ma, L.; Fan, J. Formation and transformation of schwertmannite in the classic Fenton process. J. Environ. Sci. 2019, 82, 145–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Zhou, W.; Xiao, N. Comparison of Fenton and bismuth ferrite Fenton-like pretreatments of sugarcane bagasse to enhance enzymatic saccharification. Bioresour. Technol. 2019, 285, 121343. [Google Scholar] [CrossRef]
- Hao, R.; Mao, Y.; Mao, X.; Wang, Z.; Gong, Y.; Zhang, Z.; Zhao, Y. Cooperative removal of SO2 and NO by using a method of UV-heat/H2O2 oxidation combined with NH4OH-(NH4)2SO3 dual-area absorption. Chem. Eng. J. 2019, 365, 282–290. [Google Scholar] [CrossRef]
- Song, Z.; Wang, B.; Yu, J.; Ma, C.; Chen, T.; Yang, W.; Liu, S.; Sun, L. Effect of Ti doping on heterogeneous oxidation of NO over Fe3O4 (111) surface by H2O2: A density functional study. Chem. Eng. J. 2018, 354, 517–524. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, S.; He, S.; Xiong, Y. Follow-up mechanism study on NO oxidation with vaporized H2O2 catalyzed by Fe2O3 in a fixed-bed reactor. Chem. Eng. J. 2019, 356, 662–672. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, Y. Elimination of Nitric Oxide Using New Fenton Process Based on Synergistic Catalysis: Optimization and Mechanism. Chem. Eng. J. 2019, 372, 92–98. [Google Scholar] [CrossRef]
- Yi, Z.; Wen, X.; Guo, T.; Zhou, J.; Fuproc, J.; Process, W. Desulfurization and denitrogenation from flue gas using Fenton reagent. Fuel Process. Technol. 2014, 128, 54–60. [Google Scholar]
- Cheng, L.; Huang, J.; Ni, F. Generation kinetics of hydroxyl radicals by Fenton’s reagent. Tech. Equip. Environ. Pollut. Control 2003, 12, 189–195. [Google Scholar]
- Guo, R.T.; Pan, W.G.; Zhang, X.B.; Ren, J.X.; Qiang, J.; Xu, H.J.; Jiang, W. Removal of NO by using Fenton reagent solution in a lab-scale bubbling reactor. Fuel 2011, 90, 3295–3298. [Google Scholar] [CrossRef]
- Cui, R.; Yang, B.; Li, S.; Wang, J.; Ma, S. Heterogeneous Fenton catalysts prepared from modified-fly ash for NOx removal with H2O2. Catal. Commun. 2019, 119, 180–184. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Y.; Wang, T.; Sun, Z.; Fang, C. Simultaneous removal of SO2 and NO from flue gas using iron-containing polyoxometalates as heterogeneous catalyst in UV-Fenton-like process. Fuel 2019, 250, 42–51. [Google Scholar] [CrossRef]
- Yuan, P.; Mei, X.; Shen, B.; Lu, F.; Zhou, W.; Si, M.; Chakraborty, S. Oxidation of NO by in situ Fenton reaction system with dual ions as reagents. Chem. Eng. J. 2018, 351, 660–667. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Brylewska, K.; Tarach, K.A.; Rutkowska, M.; Jabłońska, M.; Choi, M.; Chmielarz, L. IR studies of Fe modified ZSM-5 zeolites of diverse mesopore topologies in the terms of their catalytic performance in NH3-SCR and NH3-SCO processes. Appl. Catal. B: Environ. 2015, 179, 589–598. [Google Scholar] [CrossRef]
- Schiavoni, M.; Campisi, S.; Gervasini, A. Effect of Cu deposition method on silico aluminophosphate catalysts in NH3-SCR and NH3-SCO reactions. Appl. Catal. A: Gen. 2017, 543, 162–172. [Google Scholar] [CrossRef]
- Shi-Gu, Q.; Wang, Y.; Li, X. Effect of praseodymium substitution on La1-xPrxMnO3 (x = −0.4) perovskites and catalytic activity for NO oxidation. J. Phys. Chem. Solids 2019, 133, 52–58. [Google Scholar]
- Wang, H.; Xu, R.; Jin, Y.; Zhang, R. Zeolite structure effects on Cu active center, SCR performance and stability of Cu-zeolite catalysts. Catal. Today 2019, 327, 295–307. [Google Scholar] [CrossRef]
- Yu, S.; Lu, Y.; Gao, F.; Dong, L. Study on the Crystal Plane Effect of CuO/TiO2 Catalysts in NH3-SCR Reaction. Catal. Today 2019, 327, 235–245. [Google Scholar] [CrossRef]
- Ling, L.; Cao, Y.; Zhao, Z.; Liu, P.; Wang, B.; Zhang, R.; Li, D. Density functional theory calculations and analysis for the reduction of NO by H2 on Pd6/TiO2. Comput. Mater. Sci. 2018, 149, 182–190. [Google Scholar] [CrossRef]
- Jung, Y.; Pyo, Y.D.; Jang, J.; Kim, G.C.; Cho, C.P.; Yang, C. NO, NO2 and N2O emissions over a SCR using DOC and DPF systems with Pt reduction. Chem. Eng. J. 2019, 369, 1059–1067. [Google Scholar] [CrossRef]
- Locci, C.; Vervisch, L.; Farcy, B.; Domingo, P.; Perret, N. Selective Non-catalytic Reduction (SNCR) of Nitrogen Oxide Emissions: A Perspective from Numerical Modeling. Flow Turbul. Combust. 2018, 100, 301–340. [Google Scholar] [CrossRef]
- Hao, J.; Yu, W.; Lu, P.; Zhang, Y.; Zhu, X. The effects of Na/K additives and flyash on NO reduction in a SNCR process. Chemosphere 2015, 122, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.L.; Song, Q.; Yao, Q. Influence of CaO on urea pyrolysis in the selective non-catalytic reduction deNOx process. J. Anal. Appl. Pyrolysis 2017, 126, 397–404. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.Z.; Fan, S.; Hong, L.; Wang, D. SNCR De-NOx within a moderate temperature range using urea-spiked hydrazine hydrate as reductant. Chemosphere 2016, 161, 208–218. [Google Scholar] [CrossRef]
- Kang, Z.; Yuan, Q.; Zhao, L.; Dai, Y.; Sun, B.; Wang, T. Study of the performance, simplification and characteristics of SNCR de-NOx in large-scale cyclone separator. Appl. Therm. Eng. 2017, 123, 635–645. [Google Scholar] [CrossRef]
- Fu, S.L.; Song, Q.; Yao, Q. Mechanism study on the adsorption and reactions of NH3, NO, and O2 on the CaO surface in the SNCR deNOx process. Chem. Eng. J. 2016, 285, 137–143. [Google Scholar] [CrossRef]
- Fu, S.l.; Song, Q.; Yao, Q. Study on the catalysis of CaCO3 in the SNCR deNOx process for cement kilns. Chem. Eng. J. 2015, 262, 9–17. [Google Scholar] [CrossRef]
- Tan, L.; Guo, Y.; Liu, Z.; Feng, P.; Li, Z. An investigation on the catalytic characteristic of NOx reduction in SCR systems. J. Taiwan Inst. Chem. Eng. 2019, 65, 236–246. [Google Scholar]
- Odenbrand, C.U.I. CaSO4 deactivated V2O5-WO3/TiO2 SCR catalyst for a diesel power plant. Characterization and simulation of the kinetics of the SCR reactions. Appl. Catal. B: Environ. 2018, 234, 365–377. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Z.; Zhang, H.; Yang, Z. Influenced factors study and evaluation for SO2/SO3 conversion rate in SCR process. Fuel 2019, 245, 528–533. [Google Scholar] [CrossRef]
- Yu, S.; Lu, Y.; Cao, Y.; Wang, J.; Sun, B.; Gao, F.; Tang, C.; Dong, L. Composite catalytic systems: A strategy for developing the low temperature NH3-SCR catalysts with satisfactory SO2 and H2O tolerance. Catal. Today 2019, 327, 235–245. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Wang, J.; Gu, T. Improvement of activity, selectivity and H2O&SO2-tolerance of micro-mesoporous CrMn2O4 spinel catalyst for low-temperature NH3-SCR of NOx. Appl. Surf. Sci. 2019, 466, 411–424. [Google Scholar]
- Guo, M.; Liu, Q.; Zhao, P.; Han, J.; Li, X.; Ha, Y.; Fu, Z.; Song, C.; Ji, N.; Liu, C.; et al. Promotional effect of SO2 on Cr2O3 catalysts for the marine NH3-SCR reaction. Chem. Eng. J. 2019, 361, 830–838. [Google Scholar] [CrossRef]
- Niu, C.; Niu, J.; Wang, S.; Wang, Z.; Dong, S.; Fan, H.; Hong, Y.; Liu, D. Synergistic effect in one-stage dielectric barrier discharge plasma and Ag/Al2O3 catalytic systems on C2H2-SCR of NOx. Catal. Commun. 2019, 123, 49–53. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.T.; Liu, S.W.; Liu, J.; Pan, W.G.; Shi, X.; Qin, H.; Wang, Z.Y.; Qiu, Z.Z.; Liu, X.Y. The promoted performance of CeO2 catalyst for NH3-SCR reaction by NH3 treatment. Appl. Surf. Sci. 2018, 462, 187–193. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yang, R.T.; Mo, J.; Li, J.; Hao, J. The poisoning effects of calcium on V2O5-WO3/TiO2 catalyst for the SCR reaction: Comparison of different forms of calcium. Mol. Catal. 2017, 434, 16–24. [Google Scholar] [CrossRef]
- Jung, Y.; Shin, Y.J.; Pyo, Y.D.; Cho, C.P.; Jang, J.; Kim, G. NOx and N2O emissions over a Urea-SCR system containing both V2O5-WO3/TiO2 and Cu-zeolite catalysts in a diesel engine. Chem. Eng. J. 2017, 326, 853–862. [Google Scholar] [CrossRef]
- Liang, Q.M.; Li, J.; He, H.; Liang, W.J.; Zhang, T.J.; Fan, X. Effects of SO2 on the low temperature selective catalytic reduction of NO by NH3 over CeO2-V2O5-WO3/TiO2 catalysts. Front. Environ. Sci. Eng. 2017, 11, 4. [Google Scholar] [CrossRef]
- Li, P.; Liu, Q.; Liu, Z. Behaviors of NH4HSO4 in SCR of NO by NH3 over different cokes. Chem. Eng. J. 2012, 181, 169–173. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Zheng, C.; Cen, K.; Gao, X. Mechanistic investigation of enhanced reactivity of NH4HSO4 and NO on Nb- and Sb-doped VW/Ti SCR catalysts. Appl. Catal. A: Gen. 2018, 549, 310–319. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Dong, G. Mn-Ce-V-WOx/TiO2 SCR Catalysts: Catalytic Activity, Stability and Interaction among Catalytic Oxides. Catalysts 2018, 8, 76. [Google Scholar] [CrossRef]
- Tarot, M.L.; Barreau, M.; Duprez, D.; Lauga, V.; Iojoiu, E.; Courtois, X.; Can, F. Influence of the Sodium Impregnation Solvent on the Deactivation of Cu/FER-Exchanged Zeolites Dedicated to the SCR of NOx with NH3. Catalysts 2018, 8, 3. [Google Scholar] [CrossRef]
- Dong, L.; Fan, Y.; Ling, W.; Yang, C.; Huang, B. Effect of Ce/Y Addition on Low-Temperature SCR Activity and SO2 and H2O Resistance of MnOx/ZrO2/MWCNTs Catalysts. Catalysts 2017, 7, 181. [Google Scholar] [CrossRef]
- Shi, C.; Chang, H.; Wang, C.; Zhang, T.; Peng, Y.; Li, M.; Wang, Y.; Li, J. Improved Activity and H2O Resistance of Cu-Modified MnO2 Catalysts for NO Oxidation. Ind. Eng. Chem. Res. 2018, 57, 920–926. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Wang, X.; Dan, J.; Zhang, J.; Cao, P.; Dai, B. Microspherical MnO2-CeO2-Al2O3 mixed oxide for monolithic honeycomb catalyst and application in selective catalytic reduction of NOx with NH3 at 50–150 °C. Chem. Eng. J. 2018, 346, 182–192. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.-W.; Fan, Z.; Gao, G.; Niu, C. Sulfur and Water Resistance of Mn-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NOx: A Review. Catalysts 2018, 8, 11. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, F.; Zhu, M.; Dan, J.; Wang, X.; Zhang, J.; Dai, B. Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3/Vermiculite Monolithic Honeycomb Catalysts. Catalysts 2018, 8, 100. [Google Scholar] [CrossRef]
- Yu, T.; Hao, T.; Fan, D.; Wang, J.; Shen, M.; Li, W. Recent NH3-SCR mechanism research over Cu/SAPO-34 catalyst. J. Phys. Chem. C 2014, 118, 6565–6575. [Google Scholar] [CrossRef]
- Woo, J.; Leistner, K.; Bernin, D.; Ahari, H.; Shost, M.; Zammit, M.; Olsson, L. Effect of various structure directing agents (SDAs) on low-temperature deactivation of Cu/SAPO-34 during NH3-SCR reaction. Catal. Sci. Technol. 2018, 8, 3–4. [Google Scholar] [CrossRef]
- Ryu, T.; Kim, H.; Hong, S.B. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13. Appl. Catal. B: Environ. 2019, 245, 513–521. [Google Scholar] [CrossRef]
- Campisi, S.; Galloni, M.G.; Bossola, F.; Gervasini, A. Comparative performance of copper and iron functionalized hydroxyapatite catalysts in NH3-SCR. Catal. Commun. 2019, 123, 79–85. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Liu, F.; Wang, Z.; Ding, J.; Huang, H. Reaction mechanism for NH3-SCR of NOx over CuMn2O4 catalyst. Chem. Eng. J. 2019, 361, 578–587. [Google Scholar] [CrossRef]
- Wu, X.; Wang, R.; Du, Y.; Zou, C.; Meng, H.; Xie, X. Performance enhancement of NH3-SCR via employing hydrotalcite-like precursor to induce the decoration of NiO by TiO2 phase. Mol. Catal. 2019, 467, 150–160. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, Z.; Lu, P.; Xing, Y.; Wei, J.; Yue, H.; Li, R. Simultaneous oxidation of Hg0 and NH3-SCR of NO by nanophase CexZryMnzO2 at low temperature: The interaction and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 14471–14485. [Google Scholar] [CrossRef]
- Xu, H.; Zan, Q.; Zong, C.; Quan, F.; Jian, M.; Yan, N. Catalytic oxidation and adsorption of Hg0 over low-temperature NH3-SCR LaMnO3 perovskite oxide from flue gas. Appl. Catal. B Environ. 2016, 186, 30–40. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Yu, F.; Zhou, X.; Zhang, J.; Yang, S.; Dan, J.; Cao, P.; Dai, B.; Wang, Q.; et al. Mn-Ce-Fe-Al mixed oxide nanoparticles via a high shear mixer facilitated coprecipitation method for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A: Gen. 2019, 586, 117237. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.Y.; Tang, C.J.; Dong, L.; Dai, B. Both powdered and performed of MnOx-CeO2-Al2O3 catalysts synthesized by self-propagating high-temperature synthesis for the selective catalystic reduction of NOx with NH3. ACS Omega 2018, 3, 5692–5703. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, W.; Zhao, Y. New insight into hydroxyl-mediated NH3 formation on the Rh-CeO2 catalyst surface during catalytic reduction of NO by CO. Chin. J. Catal. 2017, 38, 1399–1405. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Yang, J.; Zhang, J.; Zheng, C. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas. Chem. Eng. J. 2018, 348, 618–629. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Shi, Y.; Dan, J.; Lv, Y.; Guo, X.; Dai, B. Up-scaled flash nano-precipitation production route to develop a MnOx–CeO2–Al2O3 catalyst with enhanced activity and H2O resistant performance for NOx selective catalytic reduction with NH3. Chem. Eng. Res. Des. 2018, 134, 476–486. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, L.; Cao, F.; Qian, K.; Su, S.; Hu, S.; Wang, Y.; Liu, L. Adsorption properties of NO and NH3 over MnOx based catalyst supported on γ-Al2O3. Chem. Eng. J. 2016, 302, 570–576. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, Q.; Sun, W.; Tade, M.; Liu, S. Improved activity of W-modified MnOx–TiO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2016, 288, 216–222. [Google Scholar] [CrossRef]
- Zhang, P.; Pan, W.G.; Guo, R.T.; Zhu, X.B.; Liu, J.; Qin, L.; She, X.L. The Mo modified Ce/TiO2 catalyst for simultaneous Hg0 oxidation and NO reduction. J. Energy Inst. 2018, 44, 224–254. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, C.; Yu, F.; Shi, Y.; Cao, P.; Dan, J.; Chen, K.; Lv, Y.; Guo, X.; Dai, B. Enhanced Oxygen Vacancies in a Two-Dimensional MnAl-Layered Double Oxide Prepared via Flash Nanoprecipitation Offers High Selective Catalytic Reduction of NOx with NH3. Nanomaterials 2018, 8, 620. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Tang, C.; Zhang, K.; Zhao, D.; Dong, L.; Dai, B. Highly selective catalytic reduction of NOx by MnOx–CeO2–Al2O3 catalysts prepared by self-propagating high-temperature synthesis. J. Environ. Sci. 2019, 75, 124–135. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Yuan, L.; Hang, S.; Ma, L. WO3 promoted Mn–Zr mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Acs Appl Mater. Interfaces 2016, 283, 1044–1050. [Google Scholar]
- Shin, B.; Chun, H.H.; Cha, J.S.; Shin, M.C.; Lee, H. Physico-Chemical Property and Catalytic Activity of a CeO2-Doped MnO(x)-TiO2 Catalyst with SO2 Resistance for Low-Temperature NH3-SCR of NO(x). J. Nanosci. Nanotechnol. 2016, 16, 4370. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chao, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.Y.; Hu, S.; Sun, L.S.; Zhang, A.C. The activity and mechanism study of Fe-Mn-Ce/gamma-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiu, C.; Xu, H.; Tao, L.; Lin, Z.; Gong, M.; Chen, Y. Low-temperature selective catalytic reduction of NO with NH3 over monolith catalyst of MnOx/CeO2–ZrO2–Al2O3. Catal. Today 2012, 175, 171–176. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, K.; Wang, W.; Wang, F.; Dan, J.; Yang, S.; Zhang, J.; Dai, B.; Yu, F. Enhanced selective catalytic reduction of NO with NH3 via porous micro-spherical aggregates of Mn–Ce–Fe–Ti mixed oxide nanoparticles. Green Energy Environ. 2019, 4, 311–321. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Y.; Wang, Z.; Ma, C. Comparative study of coal based catalysts for NO adsorption and NO reduction by CO. Fuel 2018, 214, 230–241. [Google Scholar] [CrossRef]
- Shen, B.; Zhu, S.; Zhang, X.; Chi, G.; Patel, D.; Si, M.; Wu, C. Simultaneous removal of NO and Hg0 using Fe and Co co-doped Mn-Ce/TiO2 catalysts. Fuel 2018, 224, 241–249. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, D. Modeling the role of CO and C3H6 in NOx reduction on a Cu-CHA SCR catalyst. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Zheng, Y.; Harold, M.P.; Luss, D. Effects of CO, H2 and C3H6 on Cu-SSZ-13 catalyzed NH3-SCR. Catal. Today 2016, 264, 44–54. [Google Scholar] [CrossRef]
- Liu, T.; Qian, J.; Yao, Y.; Shi, Z.; Han, L.; Liang, C.; Li, B.; Dong, L.; Fan, M.; Zhang, L. Research on SCR of NO with CO over the Cu0.1La0.1Ce0.8O mixed-oxide catalysts: Effect of the grinding. Mol. Catal. 2017, 430, 43–53. [Google Scholar] [CrossRef]
- Boningari, T.; Pavani, S.M.; Ettireddy, P.R.; Chuang, S.S.C.; Smirniotis, P.G. Mechanistic investigations on NO reduction with CO over Mn/TiO2 catalyst at low temperatures. Mol. Catal. 2018, 451, 33–42. [Google Scholar] [CrossRef]
- Panahi, P.N.; Salari, D.; Niaei, A.; Mousavi, S.M. NO reduction over nanostructure M-Cu/ZSM-5 (M: Cr, Mn, Co and Fe) bimetallic catalysts and optimization of catalyst preparation by RSM. J. Ind. Eng. Chem. 2013, 19, 1793–1799. [Google Scholar] [CrossRef]
- Yang, D.; Fan, X.; Zhao, D.; An, Y.; Hu, Y.; Luo, Z. Sc2CO2 and Mn-doped Sc2CO2 as gas sensor materials to NO and CO: A first-principles study. Phys. E: Low-Dimens. Syst. Nanostruct. 2019, 111, 84–90. [Google Scholar] [CrossRef]
- Chen, X.; Lyu, Y.; Nwabara, U.; Schwank, J.W. Reactivity study of CO+NO reaction over Pd/Al2O3 and Pd/CeZrO2 catalysts. Catal. Today 2019, 323, 148–158. [Google Scholar] [CrossRef]
- Cheng, G.; Tan, X.; Song, X.; Chen, X.; Dai, W.; Yuan, R.; Fu, X. Visible light assisted thermocatalytic reaction of CO + NO over Pd/LaFeO3. Appl. Catal. B: Environ. 2019, 251, 130–142. [Google Scholar] [CrossRef]
- Lopes, D.; Zotin, F.; Palacio, L.A. Copper-nickel catalysts from hydrotalcite precursors: The performance in NO reduction by CO. Appl. Catal. B: Environ. 2018, 237, 327–338. [Google Scholar] [CrossRef]
- Bánsági, T.; Zakar, T.S.; Solymosi, F. An FTIR study on the formation of NCO surface complexes over Rh/CeO2. Appl. Catal. B: Environ. 2006, 66, 147–150. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Wang, Z.; Huang, H.; Ma, C.; Qin, Y. Effect of the NO + CO reaction on the consumption of carbon supports: An in situ TG-FTIR analysis. Chem. Eng. J. 2018, 352, 90–102. [Google Scholar] [CrossRef]
- Yamashita, S.; Yamamoto, Y.; Kawabata, H.; Niwa, Y.; Katayama, M.; Inada, Y. Dynamic chemical state conversion of nickel species supported on silica under CO–NO reaction conditions. Catal. Today 2018, 303, 33–39. [Google Scholar] [CrossRef]
- Yao, X.; Li, L.; Zou, W.; Yu, S.; An, J.; Li, H.; Yang, F.; Dong, L. Preparation, characterization, and catalytic performance of high efficient CeO2-MnOx-Al2O3 catalysts for NO elimination. Chin. J. Catal. 2016, 37, 1369–1380. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Sui, G.; Tao, X.; Pan, Y.; Zhu, S. Synergistic catalytic removals of NO, CO and HC over CeO2 modified Mn-Mo-W-Ox/TiO2-SiO2 catalyst. J. Rare Earths 2018, 36, 148–155. [Google Scholar] [CrossRef]
- Xu, G.; Ma, J.; Wang, L.; Xie, W.; Liu, J.; Yu, Y.; He, H. Insight into the origin of sulfur tolerance of Ag/Al2O3 in the H2-C3H6-SCR of NOx. Appl. Catal. B: Environ. 2019, 244, 909–918. [Google Scholar] [CrossRef]
- Thomas, C. On an additional promoting role of hydrogen in the H2-assisted C3H6-SCR of NOx on Ag/Al2O3: A lowering of the temperature of formation–decomposition of the organo-NOx intermediates? Appl. Catal. B: Environ. 2015, 162, 454–462. [Google Scholar] [CrossRef]
- Xu, G.; Ma, J.; He, G.; Yu, Y.; He, H. An alumina-supported silver catalyst with high water tolerance for H2 assisted C3H6-SCR of NOx. Appl. Catal. B: Environ. 2017, 207, 60–71. [Google Scholar] [CrossRef]
- Chaieb, T.; Delannoy, L.; Costentin, G.; Louis, C.; Casale, S.; Chantry, R.L.; Li, Z.Y.; Thomas, C. Insights into the influence of the Ag loading on Al2O3 in the H2-assisted C3H6-SCR of NOx. Appl. Catal. B: Environ. 2014, 156, 192–201. [Google Scholar] [CrossRef]
- Yang, X.; Su, Y.X.; Qian, W.Y.; Yuan, M.H.; Zhou, H.; Deng, W.Y.; Zhao, B.T. Experimental study on selective catalytic reduction of NO by C3H6 over Fe-Ag/Al2O3 catalysts. J. Fuel Chem. Technol. 2017, 45, 1365–1375. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yu, H.; Wang, X. The functions of Pt located at different positions of HZSM-5 in H2-SCR. Chem. Eng. J. 2019, 355, 470–477. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, W.; Wang, Q.; Cao, L.; Yang, J. Sparsely loaded Pt/MIL-96(Al) MOFs catalyst with enhanced activity for H2-SCR in a gas diffusion reactor under 80 °C. Chem. Eng. J. 2018, 335, 612–620. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhao, X.; Xu, Y.; Liu, Y.; Yu, Q. Promotion effect of tungsten on the activity of Pt/HZSM-5 for H2-SCR. Chem. Eng. J. 2015, 260, 419–426. [Google Scholar] [CrossRef]
- Väliheikki, A.; Petallidou, K.C.; Kalamaras, C.M.; Kolli, T.; Huuhtanen, M.; Maunula, T.; Keiski, R.L.; Efstathiou, A.M. Selective catalytic reduction of NOx by hydrogen (H2-SCR) on WOx-promoted CezZr1-zO2 solids. Appl. Catal. B: Environ. 2014, 156, 72–83. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Chu, W.; Song, Z.; Zhao, S. An investigation of the surface intermediates of H2-SCR of NOx over Pt/H-FER. Appl. Catal. B: Environ. 2011, 107, 380–385. [Google Scholar] [CrossRef]
- Huai, L.Y.; He, C.Z.; Wang, H.; Wen, H.; Yi, W.C.; Liu, J.Y. NO dissociation and reduction by H2 on Pd(1 1 1): A first-principles study. J. Catal. 2015, 322, 73–83. [Google Scholar] [CrossRef]
- Liu, G.; Cui, Y.; Ji, J.; Shen, D.; Wang, Q.; Li, C.; Luo, K.H. A technical method to improve NOx/NH3 mixing ratio in SCR system and its engineering applications. J. Energy Inst. 2018, 43, 243–249. [Google Scholar] [CrossRef]
- Kikugawa, M.; Yamazaki, K.; Kato, A.; Uyama, T.; Takahashi, N.; Shinjoh, H. Silver sulfate catalyst for soot oxidation with high resistance to sulfur poisoning. Appl. Catal. A: Gen. 2019, 576, 32–38. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, J.; Liu, L.; Liu, Q.; Kong, B.; Lin, F.; Kong, M.; Hu, G. Insight into regeneration mechanism with sulfuric acid for arsenic poisoned commercial SCR catalyst. J. Energy Inst. 2019, 76, 214–223. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Walter, E.D.; Washton, N.M.; Guo, Y.; Lu, G.; Peden, C.H.F.; Gao, F. NH3-SCR on Cu, Fe and Cu+Fe exchanged beta and SSZ-13 catalysts: Hydrothermal aging and propylene poisoning effects. Catal. Today 2019, 320, 91–99. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Jensen, A.D.; Janssens, T.V.W. Impact of SO2-poisoning over the lifetime of a Cu-CHA catalyst for NH3-SCR. Appl. Catal. B: Environ. 2018, 238, 104–110. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Wang, X.; Hu, J. Activity of CuCl2-modified cobalt catalyst supported on Ti-Ce composite for simultaneous catalytic oxidation of Hg0 and NO in a simulated pre-sco process. Chem. Eng. J. 2017, 316, 1103–1113. [Google Scholar] [CrossRef]
- Salazar, M.; Hoffmann, S.; Singer, V.; Becker, R.; Grünert, W. Hybrid catalysts for the selective catalytic reduction (SCR) of NO by NH3. On the role of fast SCR in the reaction network. Appl. Catal. B: Environ. 2016, 199, 433–438. [Google Scholar] [CrossRef]
- Han, S.; Cheng, J.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Ce doping to Cu-SAPO-18: Enhanced catalytic performance for the NH3-SCR of NO in simulated diesel exhaust. Microporous Mesoporous Mater. 2019, 276, 133–146. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Lin, Q.; Hu, J.; Ran, R.; Weng, D. SO2 promoted V2O5-MoO3/TiO2 catalyst for NH3-SCR of NOx at low temperatures. Appl. Catal. A: Gen. 2019, 570, 42–50. [Google Scholar] [CrossRef]
- Langmuir, I. Oscillations in Ionized Gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahmansouri, M.; Misra, A.P. Surface plasmon oscillations in a semi-bounded semiconductor plasma. Plasma Sci. Technol. 2018, 20, 025001. [Google Scholar] [CrossRef] [Green Version]
- Parastaev, A.; Hoeben, W.F.L.M.; van Heesch, B.E.J.M.; Kosinov, N.; Hensen, E.J.M. Temperature-programmed plasma surface reaction: An approach to determine plasma-catalytic performance. Appl. Catal. B: Environ. 2018, 239, 168–177. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Sigolaeva, L.V.; Kuzikov, A.V.; Pogodin, P.V.; Archakov, A.I. Molecular imprinting coupled with electrochemical analysis for plasma samples classification in acute myocardial infarction diagnostic. Biosens. Bioelectron. 2018, 99, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Hornung, G.; Noterdaeme, J.-M.; Verdoolaege, G. Classification of ELM types in Joint European Torus based on global plasma parameters using discriminant analysis. Fusion Eng. Des. 2017, 123, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Gott, Y.V. Ion thermal conductivity in a tokamak plasma for the nonneoclassical distribution function (Review Paper). Plasma Phys. Rep. 1995, 21, 621–633. [Google Scholar]
- Capitelli, M.; Capitelli, F.; Eletskii, A. Non-equilibrium and equilibrium problems in laser-induced plasmas. Spectrochim. Acta Part. B At. Spectrosc. 2000, 55, 559–574. [Google Scholar] [CrossRef]
- Capitelli, M.; Armenise, I.; Bruno, D.; Cacciatore, M.; Celiberto, R.; Colonna, G.; Pascale, O.D.; Diomede, P.; Esposito, F.; Gorse, C. Non-equilibrium plasma kinetics: A state-to-state approach. Plasma Sources Sci. Technol. 2007, 16, S30–S44. [Google Scholar] [CrossRef]
- Lourek, I.; Tribeche, M. Dust charging current in non equilibrium dusty plasma in the context of Kaniadakis generalization. Phys. A: Stat. Mech. Appl. 2019, 517, 522–529. [Google Scholar] [CrossRef]
- Brzhozovskii, B.; Brovkova, M.; Gestrin, S.; Martynov, V.; Zinina, E. The effect of a combined low-pressure gas discharge on metal surfaces. J. Phys. D Appl. Phys. 2018, 51, 145204. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Dai, B.; Ma, C.; Yu, F. Enhanced photocatalytic degradation of organic dyes via defect-rich TiO2 prepared by dielectric barrier discharge plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Kato, T.; Takahashi, T.; Shimizu, K. Nitric oxide decomposition in air by using nonthermal plasma processing with additives and catalyst. IEEE Trans. Ind. Appl. 1998, 34, 268–272. [Google Scholar] [CrossRef]
- Baeva, M.; Gier, H.; Pott, A.; Uhlenbusch, J.; H02schele, J.; Steinwandel, J. Erratum: “Studies on Gas Purification by a Pulsed Microwave Discharge at 2.46 GHz in Mixtures of N2/NO/O2 at Atmospheric Pressure” [Plasma Chemistry and Plasma Processing, Vol. 21, No. 2, 2001]. Plasma Chem. Plasma Process. 2002, 22, 197. [Google Scholar] [CrossRef]
- Baeva, M.; Gier, H.; Pott, A.; Uhlenbusch, J.; Höschele, J.; Steinwandel, J. Pulsed microwave discharge at atmospheric pressure for NOx decomposition. Plasma Sources Sci. Technol. 2002, 11, 1–9. [Google Scholar] [CrossRef]
- Hueso, J.L.; Gonzalez-Elipe, A.R.; Cotrino, J.; Caballero, A. Plasma chemistry of NO in complex gas mixtures excited with a surfatron launcher. J. Phys. Chem. A 2005, 109, 4930–4938. [Google Scholar] [CrossRef]
- Hueso, J.L.; González-Elipe, A.R.; Cotrino, J.; Caballero, A. Removal of NO in NO/N2, NO/N2/O2, NO/CH4/N2, and NO/CH4/O2/N2 Systems by Flowing Microwave Discharges. J. Phys. Chem. A 2007, 111, 1057–1065. [Google Scholar] [CrossRef]
- Schütz, A.; Lara-Ortega, F.J.; Klute, F.D.; Brandt, S.; Schilling, M.; Michels, A.; Veza, D.; Horvatic, V.; García-Reyes, J.F.; Franzke, J. Soft Argon-Propane Dielectric Barrier Discharge Ionization. Anal. Chem. 2018, 90, 3537–3542. [Google Scholar] [CrossRef]
- Brandt, S.; Klute, F.D.; Schütz, A.; Franzke, J. Dielectric barrier discharges applied for soft ionization and their mechanism. Anal. Chim. Acta 2017, 951, 16–31. [Google Scholar] [CrossRef]
- Zuliang, W.U.; Hou, P.; Zhao, J.; Wang, J.; Yan, X.U.; Yao, S. Experimental Study of Simultaneous Desulfurization and Denitrification by Corona Discharge Coupling Wet Absorption. High. Volt. Eng. 2016, 42, 398–404. [Google Scholar]
- Chen, S.; Wang, T.; Wang, H.; Wu, Z. Insights into the reaction pathways and mechanism of NO removal by SDBD plasma via FT-IR measurements. Fuel Process. Technol. 2019, 186, 125–136. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Bogaerts, A. Propagation of a plasma streamer in catalyst pores. Plasma Sources Sci. Technol. 2018, 27, 035009. [Google Scholar] [CrossRef]
- Hong, L.; Chen, D.; Yang, M.; Yin, L.; Wang, D.; Wang, L. Interaction between NO and SO2 removal processes in a pulsed corona discharge plasma (PCDP) reactor and the mechanism. Chem. Eng. J. 2019, 359, 1130–1138. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Li, L.; Liu, L.; Meng, X.; Sun, T.; Jia, J.; Fan, M. High efficient styrene mineralization through novel NiO-TiO2-Al2O3 packed pre-treatment/treatment/post-treatment dielectric barrier discharge plasma. Chem. Eng. J. 2018, 343, 759–769. [Google Scholar] [CrossRef]
- Huang, H.; Ye, D.; Huang, B.; Wei, Z. Vanadium Supported on Viscose-Based Activated Carbon Fibers Modified By Oxygen Plasma For The Scr Of No. Catal. Today 2008, 139, 100–108. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, C.; Wu, S.; Gao, X.; Ni, M.; Cen, K. Manganese-cerium oxide catalysts prepared by non-thermal plasma for NO oxidation: Effect of O2 in discharge atmosphere. Appl. Surf. Sci. 2017, 416, 78–85. [Google Scholar] [CrossRef]
- An, J.; Jiang, Y.; Zhang, Z.; Ma, X.; Wang, T.; Shang, K.; Li, J. Oxidation characteristics of mixed NO and Hg0 in coal-fired flue gas using active species injection generated by surface discharge plasma. Chem. Eng. J. 2016, 288, 298–304. [Google Scholar] [CrossRef]
- Cui, S.; Hao, R.; Fu, D. Integrated method of non-thermal plasma combined with catalytical oxidation for simultaneous removal of SO2 and NO. Fuel 2019, 246, 365–374. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, F.; Zhou, A.; Ma, C.; Dai, B. High-efficiency removal of NOx using dielectric barrier discharge nonthermal plasma with water as an outer electrode. Plasma Sci. Technol. 2018, 20, 014020. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, R.; Xia, M.; Li, C.; Gong, X.; Wang, D.; Xia, D. Study on the mechanism of NO removal by plasma-adsorption catalytic process. Fuel 2017, 200, 290–298. [Google Scholar] [CrossRef]
- Park, H.W.; Choi, S.; Park, D.W. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with a plasma electrostatic precipitator. J. Hazard. Mater. 2015, 285, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, H.; Zhang, X.; Liu, J.; Zhang, Y.; Guo, Y.; Sun, B. Catalytic conversion of NO assisted by plasma over Mn-Ce/ZSM5-multi-walled carbon nanotubes composites: Investigation of acidity, activity and stability of catalyst in the synergic system. Appl. Surf. Sci. 2018, 457, 187–199. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Zheng, X. Plasma-assisted CuO/CeO2/TiO2-γ-Al2O3 catalysts for NO+CH4 reaction and NO temperature programmed desorption studies. Appl. Surf. Sci. 2013, 280, 273–281. [Google Scholar] [CrossRef]
- Dang, X.; Qin, C.; Huang, J.; Teng, J.; Huang, X. Adsorbed benzene/toluene oxidation using plasma driven catalysis with gas circulation: Elimination of the byproducts. J. Ind. Eng. Chem. 2016, 37, 366–371. [Google Scholar] [CrossRef]
- Tang, X.; Gao, F.; Xiang, Y.; Yi, H.; Zhao, S. Low temperature catalytic oxidation of nitric oxide over the Mn–CoOx catalyst modified by nonthermal plasma. Catal. Commun. 2015, 64, 12–17. [Google Scholar] [CrossRef]
- Niu, J.; Yang, X.; Zhu, A.; Shi, L.; Sun, Q.; Xu, Y.; Shi, C. Plasma-assisted selective catalytic reduction of NOx by C2 H2 over Co-HZSM-5 catalyst. Catal. Commun. 2006, 7, 297–301. [Google Scholar] [CrossRef]
- Broer, S.; Hammer, T. Selective catalytic reduction of nitrogen oxides by combining a non-thermal plasma and a V2O5-WO3/TiO2 catalyst. Appl. Catal. B: Environ. 2000, 28, 101–111. [Google Scholar] [CrossRef]
- Fan, H.Y.; Shi, C.; Li, X.S.; Yang, X.F.; Xu, Y.; Zhu, A.M. Low-temperature NOx Selective Reduction by Hydrocarbons on H-Mordenite Catalysts in Dielectric Barrier Discharge Plasma. Plasma Chem. Plasma Process. 2009, 29, 43–53. [Google Scholar] [CrossRef]
- Li, K.; Tang, X.; Yi, H.; Ning, P.; Song, J.; Wang, J. Mechanism of Catalytic Oxidation of NO over Mn–Co–Ce–Ox Catalysts with the Aid of Nonthermal Plasma at Low Temperature. Ind. Eng. Chem. Res. 2011, 50, 11023–11028. [Google Scholar] [CrossRef]
- Cho, B.K.; Lee, J.H.; Crellin, C.C.; Olson, K.L.; Hilden, D.L.; Kim, M.K.; Kim, P.S.; Heo, I.; Oh, S.H.; Nam, I.S. Selective catalytic reduction of NOx by diesel fuel: Plasma-assisted HC/SCR system. Catal. Today 2012, 191, 20–24. [Google Scholar] [CrossRef]
- Pan, H.; Su, Q.; Wei, J.; Jian, Y. Promotion of Nonthermal Plasma on the SO2 and H2O Tolerance of Co–In/Zeolites for the Catalytic Reduction of NOx by C3H8 at Low Temperature. Plasma Chem. Plasma Process. 2015, 35, 831–844. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Liu, J.; Liu, H.; Wang, Y.; Sun, B. Effects of temperature on NOx removal with Mn-Cu/ZSM5 catalysts assisted by plasma. Appl. Therm. Eng. 2018, 130, 1224–1232. [Google Scholar] [CrossRef]
- Tao, W.; Liu, H.; Zhang, X.; Guo, Y.; Zhang, Y.; Yang, W.; Sun, B. A plasma-assisted catalytic system for NO removal over CuCe/ZSM-5 catalysts at ambient temperature. Fuel Process. Technol. 2017, 158, 199–205. [Google Scholar]
- Jõgi, I.; Stamate, E.; Irimiea, C.; Schmidt, M.; Brandenburg, R.; Hołub, M.; Bonisławski, M.; Jakubowski, T.; Kääriäinen, M.-L.; Cameron, D.C. Comparison of direct and indirect plasma oxidation of NO combined with oxidation by catalyst. Fuel 2015, 144, 137–144. [Google Scholar]
- Li, K.; Tang, X.; Yi, H.; Ning, P.; Xiang, Y.; Wang, J.; Wang, C.; Peng, X. Research on manganese oxide catalysts surface pretreated with non-thermal plasma for NO catalytic oxidation capacity enhancement. Appl. Surf. Sci. 2013, 264, 557–562. [Google Scholar] [CrossRef]
- Garraud, O.; Coppo, P. Types of fresh plasma with focus on therapeutic plasma exchange. Transfus. Apher. Sci. 2019, 58, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimoglu, B.; Yilmazoglu, M.Z. Numerical modeling of a downdraft plasma coal gasifier with plasma reactions. Int. J. Hydrog. Energy 2019, 67, 165–173. [Google Scholar] [CrossRef]

















| Adsorbent Material | Synthetic Method | Reaction Conditions | Temperature (°C) | Conversion (%) | Ref. |
|---|---|---|---|---|---|
| Ag-Cu-BTC | preassembled method | 500 ppm NO, GHSV 10,000 h−1, N2 | 238 | 100 | [36] |
| Zeolite | Wetness impregnation | 250 mg/m NO, 1000 mg m−3 SO2 GHSV 150 L h−1, N2 | 250 | 91.7 | [62] |
| Gas-phase | microwave | 600 ppm NH3, 3% O2, and GHSV 30,000 h−1 N2 | 80 | 50 | [61] |
| ACF | Precipitation method | Flue gas 0.1–1%, GHSV 1000 mL/min, N2 | 40 | 60 | [58] |
| AC | Nitric acid hydrothermal | 1000 ppm NO, 20 vol.% O2 GHSV 16,000 h−1 N2 | 25 | 56.6 | [60] |
| Adsorbent Material | Synthetic Method | Reaction Conditions | NO Conversion % | Ref. |
|---|---|---|---|---|
| Cu/SAPO-34 | Acid–alkali hydrothermal | 300 ppm NO, 3% O2 GHSV 12,000 h−1, N2 | 90 | [63] |
| Fe/H-ZSM-5 | Hydrothermal | 5000 ppm NO, 5% O2, GHSV = 35,000 h−1, He | 63.4 | [64] |
| Fe-ZSM-5 @CeO2 | Dopamine polymerization | 1000 ppm NO, 5% O2, GHSV 33,600 h−1, N2 | 90 | [65] |
| Cu-BEA | Precipitation | 1000 ppm NO, 3.5 vol% O2 1000 mL/min, N2 | 100 | [66] |
| g-C3N4/WS2 | Solvent evaporation | 500 ppm NO, 20 vol% O2, GHSV 400 mL/min, N2 | 72.5 | [67] |
| Niobium phosphate | Impregnation | 1000 ppm NO, 1125 ppm O2, GHSV 100 mL/min, He | 100 | [68] |
| Pt/SBA-15 | Thermal hydrolysis | 4000 ppm NO, 10 vol% O2, GHSV 50 mL/min, Ar | 100 | [69] |
| Semi-coke | Hydrothermal | 1000 ppm NO, GHSV 6000 h−1, N2 | 100 | [70] |
| Sintered ore | Impregnation | 400 mg/m3 NO, 15% O2, GHSV = 1000 h−1, Ar | 61.6 | [71] |
| Oxidation Method | Reagents Materials | Temperature (°C) | NO Conversion (%) | Reference |
|---|---|---|---|---|
| Gas phase | ClO2 | 160 | 94 | [84] |
| O3 | 40 | 82 | [85] | |
| O3 | 60 | 95 | [88] | |
| Liquid phase | UV/NaClO2 | 50 | 98.1 | [92] |
| NaClO/NaClO2 | 50 | 85 | [90] | |
| NaClO2/Na2S2O8 | 120 | 82.7 | [92] | |
| Catalytic oxidation | K-OMS-2 | 50 | - | [103] |
| MnOx | 250 | 91.4 | [106] | |
| SmMn2O5 | 300 | 90 | [107] | |
| Fe0.32MnO2 | 250 | 80 | [79] | |
| Fenton | 140 | 90 | [125] | |
| La0.8Pr0.2MnO3 | 260 | 91 | [130] |
| Catalyst | Synthetic Method | GHSV (hr−1) | Temperature (°C) | Conversion (%) | Ref. |
|---|---|---|---|---|---|
| Ce-Mo/TiO2 | Coprecipitation | 19,000 | 200–400 | 90 | [177] |
| MnAl/LDO | FNP | 60,000 | 150–250 | 100 | [178] |
| MnOx-CeO2-Al2O3 | self-propagating synthesis | 15,384 | 50–400 | 100 | [179] |
| Mn–Ce-Ti | Hydrothermal | 64,000 | 150–400 | 98 | [180] |
| MnOx-CeO2-TiO2 | Sol-ge | 10,000 | 100–300 | 90 | [181] |
| Co/Ni-CeO2 | Coprecipitation | 48,000 | 75–200 | 93 | [182] |
| Fe-Mn–Ce/γ-Al2O3 | Sol-ge | 10,000 | 100–450 | 95 | [183] |
| MnOx/CeO2-ZrO2-Al2O3 | Impregnation | 10,000 | 50–300 | 90 | [184] |
| Catalyst | Synthetic Method | Reaction Conditions | Temperature (°C) | NO Conversion (%) | Ref. |
|---|---|---|---|---|---|
| Cu0.1La0.1Ce0.8O | Grind | 500 ppm NO, 1000 ppm CO, GHSV 26,000 h−1, Ar | 250 | 99.2 | [190] |
| Mn–Ce/TiO2 | wetness impregnation | 600 ppm NO, 1200 ppm CO, GHSV 40,000 h−1, N2 | 200 | 94.9 | [187] |
| Pd/CeZrO2 | Impregnation | 5 vol.% NO, 10 vol.% CO, GHSV 24,000 h−1, He | 300 | 100 | [194] |
| Pd/LaFeO3 | Two-step precipitation | 5% NO, 10% CO, Water 10%, 24,000 mL h−1, He | 120 | 98 | [195] |
| Cu-Ni/LDH | Precipitation | 1.5 vol.% CO, 0.2 vol.% NO, O2 0.65 vol.%, 12,000 h−1, He | 400 | 100 | [196] |
| Fe0.8Co0.2/ASC | Precipitation | 500 ppm NO, 1000 ppm CO, GHSV 40,000 h−1, N2 | 200 | 96 | [198] |
| Catalyst | Conversion (%) | Temperature (°C) | Ref. |
|---|---|---|---|
| NiO-TiO2-Al2O3 | Without plasma < 12 | 180–240 | [244] |
| Plasma-modified > 70 | |||
| V2O5/ACF | Without plasma < 35 | 50–150 | [245] |
| Plasma-modified > 55 |
| Catalyst | Conversion (%) | Temperature (°C) | Ref. |
|---|---|---|---|
| Co-ZSM-5 | Plasma only 12 | 150–450 | [256] |
| Catalyst only 85 | 300 | ||
| Plasma assist catalyst 95 | 300 | ||
| V2O5-WO3/TiO2 | Plasma only 20 | 150–260 | [257] |
| Catalyst only 35 | 180–260 | ||
| Plasma assist catalyst 80 | 160–260 | ||
| H-mordenite | Plasma only 3.8 | 200 | [258] |
| Catalyst only 54 | 200 | ||
| Plasma assist catalyst 91.4 | 200 | ||
| Mn-Co-CeO2 | Plasma only 73 | 150 | [259] |
| Catalyst only 70 | 150 | ||
| Plasma assist catalyst 93 | 100 | ||
| Ba & Cu (C2H2O3)2 | Plasma only 85 | 25–400 | [260] |
| Catalyst only 82 | 25–400 | ||
| Plasma assist catalyst 95 | 200 | ||
| Mn–CoOx | Plasma only 3.8 | 200 | [255] |
| Catalyst only 54 | 200 | ||
| Plasma assist catalyst 91.4 | 200 | ||
| Mn-Cu/ZSM5 | Plasma only 50 | 25–450 | [262] |
| Catalyst only 60 | 25–450 | ||
| Plasma assist catalyst 90 | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yu, F.; Ma, C.; Dan, J.; Luo, J.; Dai, B. A Critical Review of Recent Progress and Perspective in Practical Denitration Application. Catalysts 2019, 9, 771. https://doi.org/10.3390/catal9090771
Liu Z, Yu F, Ma C, Dan J, Luo J, Dai B. A Critical Review of Recent Progress and Perspective in Practical Denitration Application. Catalysts. 2019; 9(9):771. https://doi.org/10.3390/catal9090771
Chicago/Turabian StyleLiu, Zhisong, Feng Yu, Cunhua Ma, Jianming Dan, Jian Luo, and Bin Dai. 2019. "A Critical Review of Recent Progress and Perspective in Practical Denitration Application" Catalysts 9, no. 9: 771. https://doi.org/10.3390/catal9090771