Thermal Cyclic Properties of Ti-Pd-Pt-Zr High-Temperature Shape Memory Alloys
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Martensite Transformation Temperature
3.2. Thermal Cyclic Properties
3.3. Effects of Training
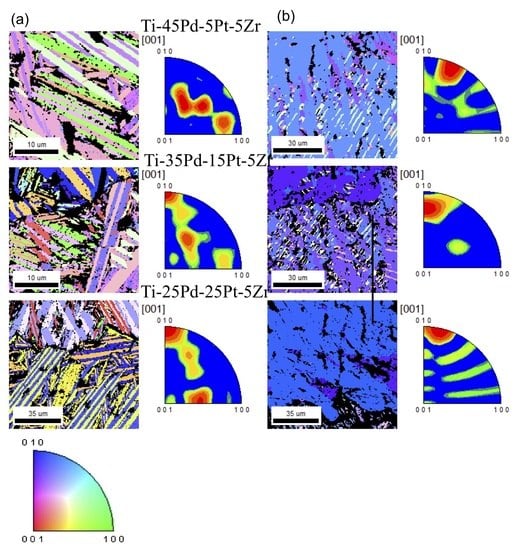
3.4. Change in Microstructure Before and After Training
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Effects of Palladium Content, Quaternary Alloying, and Thermomechanical Processing on the Behavior of Ni-Ti-Pd Shape Memory Alloys for Actuator Applications. Available online: https://ntrs.nasa.gov/search.jsp?R=20080034881 (accessed on 13 November 2019).
- Ma, J.; Karaman, I.; Noebe, R.D. High temperature shape memory alloys. Int. Mater. Review 2010, 55, 257–315. [Google Scholar] [CrossRef]
- Bastin, G.F.; Rieck, G.D. Diffusion in the titanium-nickel system: I. occurrence and growth of the various intermetallic compounds. Metall. Trans. 1974, 5, 1817–1826. [Google Scholar] [CrossRef]
- Yang, F.; Coughlin, D.R.; Phillips, P.J.; Yang, L.; Devaraj, A.; Kovarik, L. Structure analysis of a precipitate phase in an Ni-rich high-temperature NiTiHf shape memory alloy. Acta Mater. 2013, 9, 3335–3346. [Google Scholar] [CrossRef]
- Santamarta, R.; Arroyave, R.; Pons, J.; Evrigen, A.; Karaman, I.; Karacka, H.E.; Noebe, R.D. TEM study of structural and microstructural characteristics of a precipitate phase in Ni-rich Ni-Ti-Hf and Ni-Ti-Zr shape memory alloys. Acta Mater. 2013, 61, 6191–6206. [Google Scholar] [CrossRef]
- Bigelow, G.S.; Garg, A.; Padula, S.A., II; Gaydosh, D.J.; Noebe, R.D. Load-biased shape-memory and superelastic properties of a precipitation strengthened high-temperature Ni50.3Ti29.7Hf20 alloy. Scripta Mater. 2011, 64, 725–728. [Google Scholar] [CrossRef]
- Coughlin, D.R.; Phillips, P.J.; Bigelow, G.S.; Garg, A.; Noebe, R.D.; Mills, M.J. Characterization of the microstructure and mechanical properties of a 50.3Ni-29.7Ti-20Hf shape memory alloy. Scripta Mater. 2012, 67, 112–115. [Google Scholar] [CrossRef]
- Benafan, O.; Noebe, R.D.; Padula, S.A., II; Vaidyanathan, R. Microstructural Response During Isothermal and Isobaric Loading of a Precipitation-Strengthened Ni-29.7Ti-20Hf High-Temperature Shape Memory Alloy. Metall. Mater. Trans. A 2012, 43, 4539–4552. [Google Scholar] [CrossRef]
- Karaca, H.E.; Saghaian, S.M.; Ged, G.; Tobe, H.; Basaran, B.; Maier, H.J.; Noebe, R.D.; Chumlyakov, Y.I. Effects of nanoprecipitation on the shape memory and material properties of an Ni-rich NiTiHf high temperature shape memory alloy. Acta Mater. 2013, 61, 7422–7431. [Google Scholar] [CrossRef]
- Benafan, O.; Grag, A.; Noebe, R.D.; Bigelow, G.S.; Padula, S.A., II; Gaydosh, D.J.; Schell, N.; Mabe, J.H.; Vaidyanathan, R. Mechanical and functional behavior of a Ni-rich Ni50.3Ti29.7Hf20 high temperature shape memory alloy. Intermetallics 2014, 50, 94–107. [Google Scholar] [CrossRef]
- Pio, J.S.B.; Hee, Y.K.; Hideki, H.; Shuichi, M. Shape memory behavior of Ti-Ta and its potential as a high-temperature shape memory alloy. Acta Mater. 2009, 57, 1068–1077. [Google Scholar] [CrossRef]
- Joanne, L.M. The Pd-Ti (Palladium-titanium) system. Bull. Alloy Phase Diagr. 1982, 3, 321–329. [Google Scholar]
- Donkersloot, H.C.; Van Vucht, J.H.N. Martensitic transformations in gold-titanium, palladium-titanium and platinum-titanium alloys near the equiatomic composition. J. Less Common Metals 1970, 20, 83–91. [Google Scholar] [CrossRef]
- Otsuka, K.; Oda, K.; Ueno, Y.; Piao, M.; Ueki, T.; Horikawa, H. The shape memory effect in a Ti{sub 50}Pd{sub 50} alloy. Scripta Met. Mater. 1993, 29, 1355–1358. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Miura, S.; Hosoda, H. Potentials of Shape Memory Effect in (Pt, Ir)-50 at%Ti. J. Jpn. Inst. Metals 2005, 69, 634–642. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Miura, S.; Hosoda, H. Mechanical Properties of Ti-50(Pt,Ir) High-Temperature Shape Memory Alloys. Mater. Trans. 2006, 47, 650–657. [Google Scholar] [CrossRef]
- Kawakita, M.; Takahashi, M.; Takahashi, S.; Yamabe-Mitarai, Y. Effect of Zr on phase transformation and high-temperature shape memory effect in TiPd alloys. Mater Lett. 2012, 89, 336–338. [Google Scholar] [CrossRef]
- Arockiakumar, R.; Takahashi, M.; Takahashi, S.; Yamabe-Mitarai, Y. Microstructure, mechanical and shape memory properties of Ti-55Pd-5x (x=Zr, Hf, V, Nb) alloys. Mat. Sci. Eng. A 2013, 585, 85–93. [Google Scholar] [CrossRef]
- Wadood, A.; Hosoda, H.; Yamabe-Mitarai, Y. Phase transformation, oxidation and shape memory properties of Ti-50Au-10Zr alloy for high temperature applications. J. Alloy Comp. 2014, 595, 200–205. [Google Scholar] [CrossRef]
- Wadood, A.; Yamabe-Mitarai, Y. Silver- and Zirconium-added ternary and quaternary TiAu based high temperature shape memory alloys. J. Alloy Comp. 2015, 646, 1172–1177. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Miura, S.; Hosoda, H. Shape memory effect and pseudoelasticity of TiPt. Intermetallics 2010, 18, 2275–2280. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Miura, S.; Hosoda, H. Phase Transformation and Shape Memory Effect of Ti (Pt, Ir). Metall. Mater. Trans. A 2012, 43, 2901–2911. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Hara, T.; Kitashima, T.; Miura, T.; Hosoda, H. Composition dependence of phase transformation behavior and shape memory effect of Ti (Pt, Ir). J. Alloy Compd. 2013, 577, S399–S403. [Google Scholar] [CrossRef]
- Wadood, A.; Takahashi, M.; Takahashi, S.; Hosoda, H.; Yamabe-Mitarai, Y. High-temperature mechanical and shape memory properties of TiPt-Zr and TiPt-Ru alloys. Mater. Sci. Eng. A 2013, 564, 34–41. [Google Scholar] [CrossRef]
- Wadood, A.; Yamabe-Mitarai, Y. TiPt-Co and TiPt-Ru high temperature shape memory alloys. Mater. Sci. Eng. A 2014, 610, 106–110. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Wadood, A.; Arockiakumar, R.; Takahashi, M.; Takahashi, S.; Hosoda, H. High-temperature shape memory alloys based on Ti-platinum group metals compounds. Mater. Sci. Forum 2014, 783, 2541–2545. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Arockiakumar, R.; Wadood, A.; Suresh, K.S.; Kitashima, T.; Hara, T.; Shimojo, M.; Tasaki, W.; Takahashi, M.; Takahashi, S.; et al. Ti (Pt, Pd, Au) based high temperature shape memory alloys. Mater. Today: Proc. 2015, 2, S517–S522. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y. Development of High-Temperature Shape Memory Alloys above 673 K. Mater. Sci. Forum. 2017, 879, 107–112. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Takebe, W.; Shimojo, M. Phase Transformation and Shape Memory Effect of Ti-Pd-Pt–Zr High-Temperature Shape Memory Alloys. Shape Mem. Superelasticity 2017, 3, 381–391. [Google Scholar] [CrossRef]
- Takei, F.; Miura, T.; Miyazaki, S.; Kimura, S.; Otsuka, K.; Suzuki, Y. Stress-induced martensitic transformation in a Ti-Ni single crystal. Scripta Metall. 1983, 17, 987–992. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B. Effect of the atomic size distribution on glass forming ability of amorphous metallic alloys. Mater. Res. Bull. 2001, 36, 2183–2198. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D.; Maier, H.J. Comparative analysis of the effects of severe plastic deformation and thermomechanical training on the functional stability of Ti50.5Ni24.5Pd25 high-temperature shape memory alloy. Scr. Mater. 2011, 64, 315–318. [Google Scholar] [CrossRef]
- Atli, K.C.; Franco, B.E.; Karaman, I.; Gaydosh, D.; Noebe, R.D. Influence of crystallographic compatibility on residual strain of TiNi based shape memory alloys during thermo-mechanical cycling. Mater. Sci. Eng. A 2013, 574, 9–16. [Google Scholar] [CrossRef]
- Hasen, M.; Schmahl, W.W.; Hackl, K.; Heinen, R.; Frenzel, J.; Gollerthan, S.; Eggeler, G.; Wagner, M.; Khalil-Allafi, J.; Baruj, A. Hard X-ray studies of stress-induced phase transformations of superelastic NiTi shape memory alloys under uniaxial load. Mat. Sci. Eng. A 2008, 481–482, 414–419. [Google Scholar] [CrossRef]
- Jones, N.G.; Dye, D. Martensite evolution in a NiTi shape memory alloy when thermal cycling under an applied load. Intermetallics 2011, 19, 1348–1358. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. (Eds.) Pearson’s Crystal Data—Crystal Structure Database for Inorganic Compounds; ASM International(OH): Novelty, OH, USA, 2010. [Google Scholar]
- Sato, H.; Kim, Y.H.; Shimojo, M.; Yamabe-Mitarai, Y. Training Effect on Microstructure and Shape Recovery in Ti-Pd-Zr Alloys. Mater. Trans. 2017, 58, 1479–1486. [Google Scholar] [CrossRef] [Green Version]












| Alloy | As | Af | Ms | Mf | Hysteresis (Af – Ms) |
|---|---|---|---|---|---|
| Ti–50Pd–5Zr [28] | 484 | 503 | 457 | 439 | 46 |
| Ti–45Pd–5Pt–5Zr | 497 | 511 | 464 | 455 | 47 |
| Ti–35Pd–15Pt–5Zr | 539 | 557 | 485 | 471 | 72 |
| Ti–25Pd–25Pt–5Zr | 628 | 648 | 571 | 539 | 77 |
| Alloy | Ti (at%) | Zr (at%) | Pd (at%) | Pt (at%) |
|---|---|---|---|---|
| Ti-45Pd-5Pt-5Zr: Ti2Pd | 62.2 | 5.3 | 26.3 | 6.2 |
| Ti-35Pd-15Pt-5Zr: Ti2Pd | 60.6 | 7.0 | 21.3 | 11.1 |
| Ti-25Pd-25Pt-5Zr (dark): Ti3Pt | 67.7 | 1.7 | 8.6 | 22.0 |
| Ti-25Pd-25Pt-5Zr (Bright): Ti4Pt3 | 46.9 | 17.0 | 4.5 | 31.6 |
| Alloys | Martensite Phase | Austenite Phase | ||||
|---|---|---|---|---|---|---|
| Hardness at Room Temperature | Test Temperature (°C) | Proof Stress, MPa | Detwinning Stress, MPa | Detwinning Stress, MPa | Proof Stress, MPa | |
| Ti-50Pd-5Zr | 357 | 409 | 780 | - | - | 274 |
| Ti-45Pd-5Pt-5Zr | 286 | 425 | 877 | 246 | 246 | 281 |
| Ti-35Pd-15Pt-5Zr | 328 | 442 | 1080 | 387 | 387 | 315 |
| Ti-25Pd-25Pt-5Zr | 377 | 509 | 1205 | 436 | 436 | 231 |
| Ti-15Pd-35Pt-5Zr [29] | 399 | - | - | - | - | 203 |
| Ti-5Pd-45Pt-5Zr [29] | 423 | - | - | - | - | 125 |
| Ti-50Pt-5Zr [24,29] | 433 | - | - | - | - | 110 |
| Alloys | a | b | c |
|---|---|---|---|
| Ti-45Pd-5Pt-5Zr | 4.56 | 2.85 | 4.83 |
| Ti-35Pd-15Pt-5Zr | 4.6 | 2.83 | 4.85 |
| Ti-25Pd-25Pt-5Zr | 4.59 | 2.82 | 4.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasaki, W.; Shimojo, M.; Yamabe-Mitarai, Y. Thermal Cyclic Properties of Ti-Pd-Pt-Zr High-Temperature Shape Memory Alloys. Crystals 2019, 9, 595. https://doi.org/10.3390/cryst9110595
Tasaki W, Shimojo M, Yamabe-Mitarai Y. Thermal Cyclic Properties of Ti-Pd-Pt-Zr High-Temperature Shape Memory Alloys. Crystals. 2019; 9(11):595. https://doi.org/10.3390/cryst9110595
Chicago/Turabian StyleTasaki, Wataru, Masayuki Shimojo, and Yoko Yamabe-Mitarai. 2019. "Thermal Cyclic Properties of Ti-Pd-Pt-Zr High-Temperature Shape Memory Alloys" Crystals 9, no. 11: 595. https://doi.org/10.3390/cryst9110595