Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review
Abstract
:1. Introduction
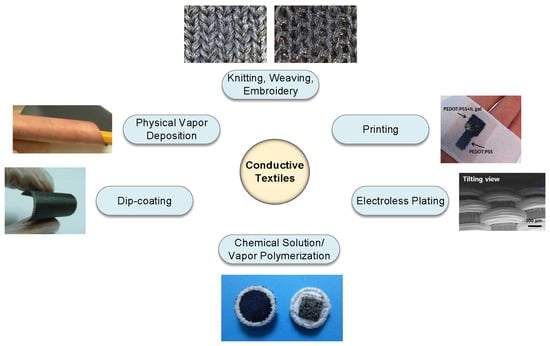
2. Materials and Methods
2.1. Knitting, Weaving, and Embroidery
2.1.1. Metallic Fibers
2.1.2. Conductive Polymer Fibers
2.2. Electrodeposition
2.3. Physical Vapor Deposition
2.4. Dip Coating
2.5. Printing
2.6. Chemical Solution/Vapor Polymerization
3. Performance Characteristics
3.1. Skin–Electrode Contact Impedance
3.2. Susceptibility to Motion Artifacts
- Measurement of an unrelated biopotential signal, for instance, electromyography (EMG) interferences in an electrocardiography (ECG) recording. Proper electrode placement can usually avoid such interference [96].
- Stretching of the skin leading to variations in the skin potential. In the textile electrode-based system, fixation of the electrodes relies on the applied pressure, which is in direct translation to the skin stretch. To reduce such motion artifacts, the applied force could be distributed to a bigger area than the electrode through the use of a supporting structure surrounding the electrode [97].
- Motion between the electrical double layer of metal and electrolyte, which causes a voltage difference in its electrochemical cell. Reducing the electrolyte resistance, polarization potential, and the movement of the electrode are believed to decrease such motion artifacts.
- Cable bending generating friction and deformation on the cable isolator, resulting in triboelectric noise [98]. To reduce this effect, a wearable garment or clothing could be designed in such a way as to secure cables and the acquisition system into the garment and provide wireless streaming of information.
- Static electricity storage and discharge caused by patient or nurse-staff localization and/or movement [99].
3.3. Stability and Lifetime
4. Conductive e-Textiles for the Acquisition of Biopotentials
4.1. Electrocardiography (ECG)
4.2. Electroencephalography (EEG)
4.3. Electromyography (EMG)
4.4. Electrooculography (EOG)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ag/AgCl | Silver/silver chloride |
| BCI | Brain computer interface |
| CNT | Carbon nanotube |
| CVD | Chemical vapor deposition |
| DSP | Digital signal processing |
| ECG | Electrocardiography |
| ECP | Extrinsically conductive polymers |
| EEG | Electroencephalography |
| EMG | Electromyography |
| EOG | Electrooculography |
| e-textile | Electronic textile |
| GO | Graphene oxide |
| HCI | Human–computer interaction |
| HMI | Human–machine interface |
| ICP | Intrinsically conductive polymers |
| ISO | International Organization of Standardization |
| mHealth | Mobile health |
| MWNT | Multi-walled carbon nanotubes |
| PANI | Polyaniline |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate |
| PET | Polyethyleneterephthalate |
| PPy | Polypyrrole |
| PU | Polyurethane |
| PVD | Physical vapor deposition |
| R&D | Research and development |
| R2R | Roll-to-roll |
| SWNT | Single-walled carbon nanotubes |
References
- Tao, X. Wearable Electronics and Photonics; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Wearable Electronics and Technology Market by Applications. Available online: http://www.marketsandmarkets.com/Market-Reports/wearable-electronics-market-983.html (accessed on 27 April 2019).
- Lee, S.; Lee, Y.; Park, J.; Choi, D. Stitchable organic photovoltaic cells with textile electrodes. Nano Energy 2014, 9, 88–93. [Google Scholar] [CrossRef]
- Khan, A.; Hussain, M.; Nur, O.; Willander, M. Fabrication of zinc oxide nanoneedles on conductive textile for harvesting piezoelectric potential. Chem. Phys. Lett. 2014, 612, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Salvado, R.; Loss, C.; Gonçalves, R.; Pinho, P. Textile Materials for the Design of Wearable Antennas: A Survey. Sensors 2012, 12, 15841–15857. [Google Scholar] [CrossRef] [Green Version]
- Hu, E.; Kaynak, A.; Li, Y. Development of a cooling fabric from conducting polymer coated fibres: Proof of concept. Synth. Met. 2005, 150, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Ostmann, A.; Vieroth, R.; Seckel, M.; Löher, T.; Reichl, H. Stretchable circuit board technology in textile applications. In Proceedings of the 2009 4th International Microsystems, Packaging, Assembly and Circuits Technology Conference, Taipei, Taiwan, 21–23 October 2009. [Google Scholar]
- Morris, D.; Coyle, S.; Wu, Y.; Lau, K.; Wallace, G.; Diamond, D. Bio-sensing textile based patch with integrated optical detection system for sweat monitoring. Sens. Actuator B Chem. 2009, 139, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Lanatà, A.; Valenza, G.; Scilingo, E. A novel EDA glove based on textile-integrated electrodes for affective computing. Med. Biol. Eng. Comput. 2012, 50, 1163–1172. [Google Scholar] [CrossRef]
- Kim, J.; Cho, G. Thermal Storage/Release, Durability, and Temperature Sensing Properties of Thermostatic Fabrics Treated with Octadecane-Containing Microcapsules. Text. Res. J. 2002, 72, 1093–1098. [Google Scholar] [CrossRef]
- Min, S.D.; Yun, Y.; Shin, H. Simplified Structural Textile Respiration Sensor Based on Capacitive Pressure Sensing Method. IEEE Sens. J. 2014, 14, 3245–3251. [Google Scholar]
- Meyer, J.; Arnrich, B.; Schumm, J.; Troster, G. Design and Modeling of a Textile Pressure Sensor for Sitting Posture Classification. IEEE Sens. J. 2010, 10, 1391–1398. [Google Scholar] [CrossRef]
- Gorgutsa, S.; Bélanger-Garnier, V.; Ung, B.; Viens, J.; Gosselin, B.; LaRochelle, S.; Messaddeq, Y. Novel Wireless-Communicating Textiles Made from Multi-Material and Minimally-Invasive Fibers. Sensors 2014, 14, 19260–19274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-D.; Chung, W.-Y. Wireless sensor network based wearable smart shirt for ubiquitous health and activity monitoring. Sens. Actuator B Chem. 2009, 140, 390–395. [Google Scholar] [CrossRef]
- Cho, J.; Moon, J.; Jeong, K.; Cho, G.J. Application of PU-sealing into Cu/Ni electroless plated polyester fabrics for e-textiles. Fibers Polym. 2007, 8, 330–334. [Google Scholar] [CrossRef]
- Carr, J.J.; Brown, J.M. Introduction to Biomedical Equipment Technology; Prentice Hall: Upper Saddle River, NJ, USA, 1993. [Google Scholar]
- Huigen, E. Noise Characteristics of Surface Electrodes. Master’s Thesis, Section Medical Physics, University of Amsterdam, Amsterdam, The Netherlands, 2001. [Google Scholar]
- Chi, Y.; Jung, T.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golparvar, A.J.; Yapici, M.K. Wearable graphene textile-enabled EOG sensing. In Proceedings of the IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017. [Google Scholar]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Jung, S.M.; Lee, C.K.; Jeong, K.S.; Cho, G.; Yoo, S.K. Wearable ECG monitoring system using conductive fabrics and active electrodes. In Proceedings of the International Conference on Human-Computer Interaction, Las Vegas, NV, USA, 15–20 July 2018. [Google Scholar]
- Qin, H.; Li, J.; He, B.; Sun, J.; Li, L.; Qian, L. Novel Wearable Electrodes Based on Conductive Chitosan Fabrics and Their Application in Smart Garments. Materials 2018, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Alzaidi, A.; Zhang, L.; Bajwa, H. Smart textiles based wireless ECG system. In Proceedings of the Systems, Applications and Technology Conference (LISAT), Farmingdale, NY, USA, 4 May 2012. [Google Scholar]
- Cho, G.; Jeong, K.; Paik, M.J.; Kwun, Y.; Sung, M. Performance evaluation of textile-based electrodes and motion sensors for smart clothing. IEEE Sens. J. 2011, 11, 3183–3193. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, X.; Zhang, J.; Duan, Y.; Hu, J.; Yang, X.J. Fabrication of conductive fabric as textile electrode for ECG monitoring. Fibers Polym. 2014, 15, 2260–2264. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-clad textile electrodes for Enabled electrocardiogram monitoring. Sens. Actuator B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lonjaret, T.; Crisp, D.; Badier, J.-M.; Malliaras, G.G.; Ismailova, E. Direct patterning of organic conductors on knitted textiles for long-term electrocardiography. Nature 2015, 5, 15003. [Google Scholar] [CrossRef] [Green Version]
- Pola, T.; Vanhala, J. Textile electrodes in ECG measurement. In Proceedings of the 2007 3rd International Conference on Intelligent Sensors, Sensor Networks and Information, Melbourne, QLD, Australia, 3–6 December 2007; pp. 635–639. [Google Scholar]
- Jang, S.; Cho, J.; Jeong, K.; Cho, G. Exploring possibilities of ECG electrodes for bio-monitoring smartwear with Cu sputtered fabrics. In Proceedings of the International Conference on Human-Computer Interaction, Bijing, China, 22–27 July 2017; pp. 1130–1137. [Google Scholar]
- Trindade, I.G.; Martins, F.; Baptista, P. High electrical conductance poly (3, 4-ethylenedioxythiophene) coatings on textile for electrocardiogram monitoring. Synth. Met. 2015, 210, 179–185. [Google Scholar] [CrossRef]
- An, X.; Stylios, G. A Hybrid Textile Electrode for Electrocardiogram (ECG) Measurement and Motion Tracking. Materials 2018, 11, 1887. [Google Scholar] [CrossRef]
- Mestrovic, M.A.; Helmer, R.J.; Kyratzis, L.; Kumar, D. Preliminary study of dry knitted fabric electrodes for physiological monitoring. In Proceedings of the Intelligent Sensors, Sensor Networks and Information, Melbourne, QLD, Australia, 3–6 December 2007. [Google Scholar]
- Xu, P.; Zhang, H.; Tao, X. Textile-structured electrodes for electrocardiogram. Text. Prog. 2008, 40, 183–213. [Google Scholar] [CrossRef]
- Xie, L.; Yang, G.; Xu, L.; Seoane, F.; Chen, Q.; Zheng, L. Characterization of dry biopotential electrodes. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC 2013, Osaka, Japan, 3–7 July 2013; pp. 1478–1481. [Google Scholar]
- Zhang, H.; Li, W.; Tao, X.; Xu, P.; Liu, H. Textile-structured human body surface biopotential signal acquisition electrode. In Proceedings of the 2011 4th International Congress on Image and Signal Processing, Shanghai, China, 15–17 October 2011; pp. 2792–2797. [Google Scholar]
- Gong, R.H.; Wright, R.M. Fancy Yarns: Their Manufacture and Application; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Apiwattanadej, T.; Zhang, L.; Li, H. Electrospun polyurethane microfiber membrane on conductive textile for water-supported textile electrode in continuous ECG monitoring application. In Proceedings of the 2018 Symposium on Design, Test, Integration & Packaging of MEMS and MOEMS (DTIP), Roma, Italy, 22–25 May 2018; pp. 1–5. [Google Scholar]
- Poggio, C.; Trovati, F.; Ceci, M.; Chiesa, M.; Colombo, M.; Pietrocola, G. Biological and antibacterial properties of a new silver fiber post: In vitro evaluation. J. Clin. Exp. Dent. 2017, 9, e387–e393. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.C.; Seoane, F.; Välimäki, E.; Lindecrantz, K. Comparison of dry-textile electrodes for electrical bioimpedance spectroscopy measurements. J. Phys. Conf. Ser. 2010, 224, 012140. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, M.J.M.; Engineering, B. Cardiopulmonary monitoring by textile electrodes without subject-awareness of being monitored. Med. Biol. Eng. Comput. 1997, 35, 685–690. [Google Scholar] [CrossRef]
- Puurtinen, M.M.; Komulainen, S.M.; Kauppinen, P.K.; Malmivuo, J.A.V.; Hyttinen, J.A.K. Measurement of noise and impedance of dry and wet textile electrodes, and textile electrodes with hydrogel. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 6012–6015. [Google Scholar]
- Catrysse, M.; Puers, R.; Hertleer, C.; Van Langenhove, L.; Van Egmond, H.; Matthys, D. Towards the integration of textile sensors in a wireless monitoring suit. Sens. Actuator A Phys. 2004, 114, 302–311. [Google Scholar] [CrossRef]
- Ali, A.; Nguyen, N.H.; Baheti, V.; Ashraf, M.; Militky, J.; Mansoor, T.; Noman, M.T.; Ahmad, S. Electrical conductivity and physiological comfort of silver coated cotton fabrics. J. Text. Inst. 2018, 109, 620–628. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Jerković, I.; Koncar, V.; Cochrane, C.; Kelly, F.M.; Soulat, D.; Legrand, X.J. Conductive polymer for smart textile applications. J. Ind. Text. 2018, 48, 612–642. [Google Scholar] [CrossRef]
- Jianming, J.; Wei, P.; Shenglin, Y.; Guang, L. Electrically conductive PANI-DBSA/Co-PAN composite fibers prepared by wet spinning. Synth. Met. 2005, 149, 181–186. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G.M.; Wallace, G.G.; Whitten, P.G. Production of polypyrrole fibres by wet spinning. Synth. Met. 2008, 158, 104–107. [Google Scholar] [CrossRef]
- Okuzaki, H.; Harashina, Y.; Yan, H.J. Highly conductive PEDOT/PSS microfibers fabricated by wet-spinning and dip-treatment in ethylene glycol. Adv. Funct. Mater. 2009, 45, 256–261. [Google Scholar] [CrossRef]
- Afshari, M. Electrospun Nanofibers, 1st ed.; Woodhead Publishing: Duxford, UK, 2017; pp. 467–519. [Google Scholar]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, W.; Wang, W.; Fong, H.; Zhu, Z. Scalable and Facile Preparation of Highly Stretchable Electrospun PEDOT: PSS@PU Fibrous Nonwovens toward Wearable Conductive Textile Applications. ACS Appl. Mater. Interfaces 2017, 9, 30014–30023. [Google Scholar] [CrossRef] [PubMed]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; William Andrew: Norwich, NY, USA, 1990. [Google Scholar]
- Cho, J.; Moon, J.; Jeong, K.; Cho, G. An exploration of electrolessly Cu/Ni plated polyester fabrics as E-textiles. In Proceedings of the Ninth IEEE International Symposium on Wearable Computers (ISWC’05), Osaka, Japan, 18–21 October 2005; pp. 206–207. [Google Scholar]
- Hegemann, D.; Amberg, M.; Ritter, A.; Heuberger, M. Recent developments in Ag metallised textiles using plasma sputtering. Adv. Perform. Mater. 2009, 24, 41–45. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Yeom, S.-W.; Oh, I.-K.J. Fabrication and actuation of ionic polymer metal composites patterned by combining electroplating with electroless plating. Compos. Part A Appl. Sci. Manuf. 2008, 39, 588–596. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; William Andrew: New York, NY, USA, 2010. [Google Scholar]
- Pawlak, R.; Korzeniewska, E.; Koneczny, C.; Hałgas, B. Properties of thin metal layers deposited on textile composites by using the PVD method for textronic applications. Autex Res. J. 2017, 17, 229–237. [Google Scholar] [CrossRef]
- Lacerda Silva, N.; Goncalves, L.M.; Carvalho, H. Deposition of conductive materials on textile and polymeric flexible substrates. J. Mater. Sci. Mater. Electron. 2012, 635–643. [Google Scholar] [CrossRef]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.; Abächerli, R.; Meyer, V.; Rossi, R. Embroidered Electrode with Silver/Titanium Coating for Long-Term ECG Monitoring. Sensors 2015, 15, 1750–1759. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Ritter, A.; Reimann, P.; Thommen, V.; Fischer, A.; Hegemann, D. Comparative study of plasma-induced and wet-chemical cleaning of synthetic fibers. Surf. Coat. Technol. 2005, 200, 1045–1050. [Google Scholar] [CrossRef]
- Shang, S.; Zeng, W. Conductive nanofibres and nanocoatings for smart textiles. In Multidisciplinary Know-How for Smart-Textiles Developers; Woodhead Publishing: Cambridge, UK, 2013; pp. 92–128. [Google Scholar]
- Tang, X.; Yan, X.J. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Ankhili, A.; Tao, X.; Cochrane, C.; Coulon, D.; Koncar, V. Washable and Reliable Textile Electrodes Embedded into Underwear Fabric for Electrocardiography (ECG) Monitoring. Materials 2018, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, Y.; Liu, J.; Zhan, Z.; Li, X.; Li, W.J. Single-Wall Carbon Nanotube-Coated Cotton Yarn for Electrocardiography Transmission. Micromachines 2018, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Sen, A. Coated Textiles; CRC Press c/o Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Garcia-Breijo, E.; Prats-Boluda, G.; Lidon-Roger, J.V.; Ye-Lin, Y.; Garcia-Casado, J. A comparative analysis of printing techniques by using an active concentric ring electrode for bioelectrical recording. Microelectron. Int. 2015, 32, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192. [Google Scholar] [CrossRef] [PubMed]
- Molina, J. Graphene-based fabrics and their applications: A review. RSC Adv. 2016, 6, 68261–68291. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Yapici, M.K. Wearable Graphene Nanotextile Embedded Smart Armband for Cardiac Monitoring. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018. [Google Scholar]
- Wu, Y.-Z.; Sun, J.-X.; Li, L.-F.; Ding, Y.-S.; Xu, H.-A. Performance evaluation of a novel cloth electrode. In Proceedings of the Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010; pp. 1–5. [Google Scholar]
- Lam, C.L.; Rajdi, N.N.Z.M.; Wicaksono, D.H. MWCNT/Cotton-based flexible electrode for electrocardiography. In Proceedings of the IEEE SENSORS, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Nature 2017, 7, 40572. [Google Scholar] [CrossRef] [Green Version]
- Yapici, M.K.; Alkhidir, T.E. Intelligent medical garments with graphene-functionalized smart-cloth ECG sensors. Sensors 2017, 17, 875. [Google Scholar] [CrossRef]
- Ujiie, H. Digital Printing of Textiles; Woodhead Publishing: Cambridge, UK, 2006. [Google Scholar]
- Hart, J.P.; Wring, S.A. Screen-printed voltammetric and amperometric electrochemical sensors for decentralized testing. Electroanalysis 1994, 6, 617–624. [Google Scholar] [CrossRef]
- Guo, Y.; Otley, M.T.; Li, M.; Zhang, X.; Sinha, S.K.; Treich, G.M.; Sotzing, G.A. PEDOT: PSS “wires” printed on textile for wearable electronics. ACS Appl. Mater. Interfaces 2016, 8, 26998–27005. [Google Scholar] [CrossRef]
- Hu, B.; Li, D.; Ala, O.; Manandhar, P.; Fan, Q.; Kasilingam, D.; Calvert, P.D.J.A.F.M. Textile-Based Flexible Electroluminescent Devices. Adv. Funct. Mater. 2011, 21, 305–311. [Google Scholar] [CrossRef]
- Karaguzel, B.; Merritt, C.; Kang, T.; Wilson, J.; Nagle, H.; Grant, E.; Pourdeyhimi, B. Utility of nonwovens in the production of integrated electrical circuits via printing conductive inks. J. Text. I 2008, 99, 37–45. [Google Scholar] [CrossRef]
- Paul, G.; Torah, R.; Beeby, S.; Tudor, J. The development of screen printed conductive networks on textiles for biopotential monitoring applications. Sens. Actuator A Phys. 2014, 206, 35–41. [Google Scholar] [CrossRef]
- Kamyshny, A.; Steinke, J.; Magdassi, S. Metal-based inkjet inks for printed electronics. Appl. Phys. Lett. 2011, 4, 19–36. [Google Scholar] [CrossRef]
- Chen, S.-P.; Chiu, H.-L.; Wang, P.-H.; Liao, Y.-C. Inkjet printed conductive tracks for printed electronics. ECS J. Solid State Sci. Technol. 2015, 4, P3026–P3033. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Ismailova, E.; Saadaoui, M.; Isik, M.; Sanchez-Sanchez, A.; Mecerreyes, D.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Fully printed electrodes on stretchable textiles for long-term electrophysiology. Adv. Mater. Technol. 2017, 2, 160025. [Google Scholar] [CrossRef]
- Skrzetuska, E.; Puchalski, M.; Krucińska, I. Chemically driven printed textile sensors based on graphene and carbon nanotubes. Sensors 2014, 14, 16816–16828. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Malandraki, A.; Butterworth, S.; Beach, C.; Rigout, M.; Novoselov, K.S.; Casson, A.J.; Yeates, S.G. All inkjet-printed graphene-based conductive patterns for wearable e-textile applications. J. Mater. Chem. 2017, 5, 11640–11648. [Google Scholar] [CrossRef] [Green Version]
- Allison, L.; Hoxie, S.; Andrew, T.L. Towards seamlessly-integrated textile electronics: Methods to coat fabrics and fibers with conducting polymers for electronic applications. Chem. Commun. 2017, 53, 7182–7193. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Byun, S.; Jeong, S.; Hong, Y.; Joo, J.; Song, K.; Kim, J.; Lee, C.; Lee, J. PET fabric/polypyrrole composite with high electrical conductivity for EMI shielding. Synth. Met. 2002, 126, 233–239. [Google Scholar] [CrossRef]
- Taji, B.; Shirmohammadi, S.; Groza, V.; Batkin, I. Impact of Skin–Electrode Interface on Electrocardiogram Measurements Using Conductive Textile Electrodes. IEEE Trans. Instrum. Meas. 2014, 63, 1412–1422. [Google Scholar] [CrossRef]
- Webster, J.G. Medical Instrumentation Application and Design; John Wiley & Sons: Temple Terrace, FL, USA, 2009. [Google Scholar]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef]
- Baba, A.; Burke, M.; Mastorakis, N. Electrical characterization of dry electrodes for ecg recording. In Proceedings of the 12th WSEAS International Conference, Heraklion, Greece, 22–24 July 2008. [Google Scholar]
- Scilingo, E.; Gemignani, A.; Paradiso, R.; Taccini, N.; Ghelarducci, B.; DeRossi, D. Performance Evaluation of Sensing Fabrics for Monitoring Physiological and Biomechanical Variables. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 345–352. [Google Scholar] [CrossRef]
- Wu, W.; Pirbhulal, S.; Sangaiah, A.K.; Mukhopadhyay, S.C.; Li, G. Optimization of signal quality over comfortability of textile electrodes for ECG monitoring in fog computing based medical applications. Future Gener. Comput. Syst. 2018, 86, 515–526. [Google Scholar] [CrossRef]
- Simakov, A.; Webster, J. Motion artifact from electrodes and cables. Iran. J. Electr. Comput. Eng. 2010, 9, 139–143. [Google Scholar]
- Priniotakis, G.; Westbroek, P.; Van Langenhove, L.; Hertleer, C. Electrochemical impedance spectroscopy as an objective method for characterization of textile electrodes. Trans. Inst. Meas. Control 2007, 29, 271–281. [Google Scholar] [CrossRef]
- Webster, J.G. Reducing motion artifacts and interference in biopotential recording. IEEE Trans. Biomed. Eng. 1984, 823–826. [Google Scholar] [CrossRef]
- Cömert, A.; Hyttinen, J. Investigating the possible effect of electrode support structure on motion artifact in wearable bioelectric signal monitoring. Biomed. Eng. Online 2015, 14, 44. [Google Scholar] [CrossRef] [Green Version]
- Ratz, A.G. Triboelectric noise (Triboelectric noise in mechanically flexed low level signal cables for piezoelectric transducers with high gain amplifiers). ISA Trans. 1969, 9, 154–158. [Google Scholar]
- Meziane, N.; Yang, S.; Shokoueinejad, M.; Webster, J.; Attari, M.; Eren, H. Simultaneous comparison of 1 gel with 4 dry electrode types for electrocardiography. Physiol. Meas. 2015, 36, 513–529. [Google Scholar] [CrossRef]
- Taji, B.; Shirmohammadi, S.; Groza, V. Measuring skin-electrode impedance variation of conductive textile electrodes under pressure. In Proceedings of the Instrumentation and Measurement Technology Conference (I2MTC), Montevideo, Uruguay, 12–15 May 2014; pp. 1083–1088. [Google Scholar]
- ISO. 6330: 2012 Textiles—Domestic Washing and Drying Procedures for Textile Testing; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Kazani, I.; Hertleer, C.; De Mey, G.; Schwarz, A.; Guxho, G.; Van Langenhove, L. Electrical conductive textiles obtained by screen printing. Fibres Text. East. Eur. 2012, 20, 57–63. [Google Scholar]
- Merritt, C.R.; Nagle, H.T.; Grant, E. Fabric-based active electrode design and fabrication for health monitoring clothing. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 274–280. [Google Scholar] [CrossRef]
- Mohammed, J.; Lung, C.-H.; Ocneanu, A.; Thakral, A.; Jones, C.; Adler, A. Internet of things: Remote patient monitoring using web services and cloud computing. In Proceedings of the 2014 IEEE International Conference on Internet of Things (iThings), and IEEE Green Computing and Communications (GreenCom) and IEEE Cyber Physical and Social Computing (CPSCom), Taipei, Taiwan, 1–3 September 2014. [Google Scholar]
- Boehm, A.; Yu, X.; Neu, W.; Leonhardt, S.; Teichmann, D. A novel 12-lead ECG T-shirt with active electrodes. mdpi electronics. Electronics 2016, 5, 75. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Yoo, J.; Yan, L. Wireless fabric patch sensors for wearable healthcare. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5254–5257. [Google Scholar]
- Silva, M.; Catarino, A.; Carvalho, H.; Rocha, A.; Monteiro, J.; Montagna, G. Study of vital sign monitoring with textile sensors in swimming pool environment. In Proceedings of the 35th Annual Conference of IEEE Industrial Electronics, Porto, Portugal, 3–5 November 2009. [Google Scholar] [CrossRef]
- Bouwstra, S.; Chen, W.; Feijs, L.; Oetomo, S.B. Smart jacket design for neonatal monitoring with wearable sensors. In Proceedings of the Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3–5 June 2009; pp. 162–167. [Google Scholar] [CrossRef]
- Coosemans, J.; Hermans, B.; Puers, R.J.S.; Physical, A.A. Integrating wireless ECG monitoring in textiles. Sens. Actuators A Phys. 2006, 130, 48–53. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Wu, T.; Zhang, Y. A wearable mobihealth care system supporting real-time diagnosis and alarm. Med. Biol. Eng. Comput. 2007, 45, 877–885. [Google Scholar] [CrossRef]
- Fleury, A.; Alizadeh, M.; Stefan, G.; Chau, T. Toward fabric-based EEG access technologies: Seamless knit electrodes for a portable brain-computer interface. In Proceedings of the 2017 IEEE Life Sciences Conference (LSC), Sydney, NSW, Australia, 13–15 December 2017; pp. 35–38. [Google Scholar] [CrossRef]
- Matiko, J.W.; Wei, Y.; Torah, R.; Grabham, N.; Paul, G.; Beeby, S.; Tudor, J. Wearable EEG headband using printed electrodes and powered by energy harvesting for emotion monitoring in ambient assisted living. Smart Mater. Struct. 2015, 24, 125028. [Google Scholar] [CrossRef]
- La, T.G.; Qiu, S.; Scott, D.K.; Bakhtiari, R.; Kuziek, J.W.; Mathewson, K.E.; Rieger, J.; Chung, H.J. Two-Layered and Stretchable e-Textile Patches for Wearable Healthcare Electronics. Adv. Healthc. Mater. 2018, 7, 1801033. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.-O.; Kang, T.; Park, J.; Choi, Y. Knit Band Sensor for Myoelectric Control of Surface EMG-based Prosthetic Hand. IEEE Sens. J. 2018. [Google Scholar] [CrossRef]
- Pino, E.J.; Arias, Y.; Aqueveque, P. Wearable EMG Shirt for Upper Limb Training. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018. [Google Scholar] [CrossRef]
- Farina, D.; Lorrain, T.; Negro, F.; Jiang, N. High-density EMG E-Textile systems for the control of active prostheses. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar] [CrossRef]
- Guo, J.; Yu, S.; Li, Y.; Huang, T.-H.; Wang, J.; Lynn, B.; Fidock, J.; Shen, C.-L.; Edwards, D.; Su, H. A soft robotic exo-sheath using fabric EMG sensing for hand rehabilitation and assistance. In Proceedings of the IEEE International Conference on Soft Robotics (RoboSoft), Livorno, Italy, 24–28 April 2018. [Google Scholar] [CrossRef]
- Zhang, R.; Bernhart, S.; Amft, O. Diet eyeglasses: Recognising food chewing using EMG and smart eyeglasses. In Proceedings of the IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016. [Google Scholar] [CrossRef]
- Nelson, A.; Schmandt, J.; Shyamkumar, P.; Wilkins, W.; Lachut, D.; Banerjee, N.; Rollins, S.; Parkerson, J.; Varadan, V. Wearable multi-sensor gesture recognition for paralysis patients. In Proceedings of the IEEE SENSORS, Baltimore, MD, USA, 3–6 November 2013. [Google Scholar]
- Liang, S.-F.; Kuo, C.-E.; Lee, Y.-C.; Lin, W.-C.; Liu, Y.-C.; Chen, P.-Y.; Cherng, F.-Y.; Shaw, F.-Z. Development of an EOG-based automatic sleep-monitoring eye mask. IEEE Trans. Instrum. Meas. 2015, 64, 2977–2985. [Google Scholar] [CrossRef]
- Golparvar, A.J.; Yapici, M.K. Electrooculography by Wearable Graphene Textiles. IEEE Sens. J. 2018, 18, 8971–8978. [Google Scholar] [CrossRef]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/cardiovascular_diseases/en/ (accessed on 25 November 2018).
- Yang, G.-Z.; Yang, G. Body Sensor Networks; Springer: London, UK, 2006; Volume 1. [Google Scholar]
- Sornmo, P.; Laguna, L. The Electrocardiogram-a Brief Background. In Bioelectrical Signal Processing in Cardiac and Neurological Applications; Elsevier Academic Press: Burlington, NJ, USA, 2005. [Google Scholar]
- Lai, D.T.H.; Palaniswami, M.; Begg, R. Healthcare Sensor Networks: Challenges toward Practical Implementation; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- López, G.; Custodio, V.; Moreno, J. LOBIN: E-textile and wireless-sensor-network-based platform for healthcare monitoring in future hospital environments. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1446–1458. [Google Scholar] [CrossRef]
- Darling, C.E.; Dovancescu, S.; Saczynski, J.S.; Riistama, J.; Kuniyoshi, F.S.; Rock, J.; Meyer, T.E.; McManus, D.D. Bioimpedance-Based Heart Failure Deterioration Prediction Using a Prototype Fluid Accumulation Vest-Mobile Phone Dyad: An Observational Study. JMIR Cardio 2017, 1, e1. [Google Scholar] [CrossRef]
- Buttussi, F.; Chittaro, L. MOPET: A context-aware and user-adaptive wearable system for fitness training. Artif. Intell. Med. 2008, 42, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Coyle, S.; Lau, K.-T.; Moyna, N.; O’Gorman, D.; Diamond, D.; Di Francesco, F.; Costanzo, D.; Salvo, P.; Trivella, M.G.; De Rossi, D.E. BIOTEX—Biosensing textiles for personalized healthcare management. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 364–370. [Google Scholar] [CrossRef]
- Pandian, P.; Mohanavelu, K.; Safeer, K.; Kotresh, T.; Shakunthala, D.; Gopal, P.; Padaki, V. Smart Vest: Wearable multi-parameter remote physiological monitoring system. Med. Eng. Phys. 2008, 30, 466–477. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Vaini, E.; Lombardi, P.J.S.; Physical, A.A. Development of a smart garment for the assessment of cardiac mechanical performance and other vital signs during sleep in microgravity. Sens. Actuators A Phys. 2018, 274, 19–27. [Google Scholar] [CrossRef]
- Linti, C.; Horter, H.; Osterreicher, P.; Planck, H. Sensory baby vest for the monitoring of infants. In Proceedings of the Wearable and Implantable Body Sensor Networks. International Workshop on Wearable and Implantable Body Sensor Networks (BSN’06), Cambridge, MA, USA, 3–5 April 2006; pp. 3–137. [Google Scholar] [CrossRef]
- Ottenbacher, J.; Romer, S.; Kunze, C.; Großmann, U.; Stork, W. Integration of a bluetooth based ECG system into clothing. In Proceedings of the Eighth International Symposium on Wearable Computers, Arlington, VA, USA, 31 October–3 November 2004. [Google Scholar] [CrossRef]
- Linz, T.; Kallmayer, C.; Aschenbrenner, R.; Reichl, H. Fully untegrated EKG shirt based on embroidered electrical interconnections with conductive yarn and miniaturized flexible electronics. In Proceedings of the International Workshop on Wearable and Implantable Body Sensor Networks (BSN’06), Cambridge, MA, USA, 3–5 April 2006. [Google Scholar] [CrossRef]
- Teplan, M. Fundamentals of EEG measurement. Meas. Sci. Technol. 2002, 2, 1–11. [Google Scholar]
- Kirschstein, T.; Köhling, R. What is the Source of the EEG? Sage Open 2009, 40, 146–149. [Google Scholar] [CrossRef]
- Guger, C.; Edlinger, G.; Harkam, W.; Niedermayer, I.; Pfurtscheller, G. How many people are able to operate an EEG-based brain-computer interface (BCI)? IEEE Eng. Med. Biol. Mag. 2003, 11, 145–147. [Google Scholar] [CrossRef]
- Casson, A.J.; Smith, S.; Duncan, J.S.; Rodriguez-Villegas, E. Wearable EEG: What is it, why is it needed and what does it entail? In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Lehnertz, K.; Richardson, M.P.; Schelter, B.; Zaveri, H.P. Seizure prediction ready for a new era. Nat. Rev. Neurol 2018, 1. [Google Scholar] [CrossRef]
- Guy, V.; Soriani, M.-H.; Bruno, M.; Papadopoulo, T.; Desnuelle, C.; Clerc, M. Brain computer interface with the P300 speller: Usability for disabled people with amyotrophic lateral sclerosis. Ann. Phys. Rehabil. Med. 2018, 61, 5–11. [Google Scholar] [CrossRef]
- Petersen, M.K.; Stahlhut, C.; Stopczynski, A.; Larsen, J.E.; Hansen, L.K. Smartphones get emotional: Mind reading images and reconstructing the neural sources. In Proceedings of the International Conference on Affective Computing and Intelligent Interaction, Memphis, TN, USA, 9–12 October 2011. [Google Scholar] [CrossRef]
- Mesárošová, A.; Hernández, M.F.; Mesároš, P.; Behún, M. Mixing augmented reality and EEG technology to create an unique learning tool for construction process. In Proceedings of the 2017 15th International Conference on Emerging eLearning Technologies and Applications (ICETA), Stary Smokovec, Slovakia, 26–27 October 2017; pp. 1–7. [Google Scholar] [CrossRef]
- Simic, M.; Tariq, M.; Trivailo, P.M. EEG-Based BCI Control Schemes for Lower-Limb Assistive-Robots. Front. Hum. Neurosci. 2018, 12, 312. [Google Scholar] [CrossRef]
- Chan, H.-L.; Kuo, P.-C.; Cheng, C.-Y.; Chen, Y. Challenges and Future Perspectives on Electroencephalogram-Based Biometrics in Person Recognition. Front. Neuroinform. 2018, 12. [Google Scholar] [CrossRef]
- Arnin, J.; Anopas, D.; Horapong, M.; Triponyuwasi, P.; Yamsa-ard, T.; Iampetch, S.; Wongsawat, Y. Wireless-based portable EEG-EOG monitoring for real time drowsiness detection. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4977–4980. [Google Scholar] [CrossRef]
- Löfhede, J.; Seoane, F.; Thordstein, M.J.S. Textile electrodes for EEG recording—A pilot study. Sensors 2012, 12, 16907–16919. [Google Scholar] [CrossRef]
- Lin, C.-T.; Liao, L.-D.; Liu, Y.-H.; Wang, I.-J.; Lin, B.-S.; Chang, J.-Y. Novel dry polymer foam electrodes for long-term EEG measurement. IEEE Eng. Med. Biol. Mag. 2011, 58, 1200–1207. [Google Scholar] [CrossRef]
- Garcia, M.C.; Vieira, T.M.M. Surface electromyography: Why, when and how to use it. Rev. Andal. Med. Deport. 2011, 4, 17–28. [Google Scholar]
- Mathiassen, S.; Winkel, J.; Hägg, G. Normalization of surface EMG amplitude from the upper trapezius muscle in ergonomic studies—A review. J. Electromyogr. Kinesiol. 1995, 5, 197–226. [Google Scholar] [CrossRef]
- Castellini, C.; Smagt, P.V.D. Surface EMG in advanced hand prosthetics. Biol. Cybern. 2008, 100, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Turker, H.; Soze, H. Surface Electromyography in Sports and Exercise. In Electrodiagnosis in New Frontiers of Clinical Research; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Al-Mulla, M.R.; Sepulveda, F.; Colley, M. A Review of Non-Invasive Techniques to Detect and Predict Localised Muscle Fatigue. Sensors 2011, 11, 3545–3594. [Google Scholar] [CrossRef] [Green Version]
- Shafti, A.; Manero, R.B.R.; Borg, A.M.; Althoefer, K.; Howard, M.J. Embroidered Electromyography: A Systematic Design Guide. IEEE Eng. Med. Biol. Mag. 2017, 25, 1472–1480. [Google Scholar] [CrossRef]
- Nishimura, S.; Tomita, Y.; Horiuchi, T. Clinical application of an active electrode using an operational amplifier. IEEE Eng. Med. Biol. Mag. 1992, 39, 1096–1099. [Google Scholar] [CrossRef]
- Cömert, A.; Honkala, M.; Hyttinen, J. Effect of pressure and padding on motion artifact of textile electrodes. Biomed. Eng. 2013. [Google Scholar] [CrossRef]
- Sumner, B.; Mancuso, C.; Paradiso, R. Performances evaluation of textile electrodes for EMG remote measurements. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar] [CrossRef]
- Niijima, A.; Isezaki, T.; Aoki, R.; Watanabe, T.; Yamada, T. hitoeCap: Wearable EMG sensor for monitoring masticatory muscles with PEDOT-PSS textile electrodes. In Proceedings of the 2017 ACM International Symposium on Wearable Computers—ISWC 17, Maui, Hawaii, 11–15 September 2017. [Google Scholar] [CrossRef]
- Li, G.; Geng, Y.; Tao, D.; Zhou, P. Performance of electromyography recorded using textile electrodes in classifying arm movements. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar] [CrossRef]
- Tikkanen, O.; Kärkkäinen, S.; Haakana, P.; Kallinen, M.; Pullinen, T.; Finni, T. EMG, Heart Rate, and Accelerometer as Estimators of Energy Expenditure in Locomotion. Med. Sci. Sports Exerc. 2014, 46, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, O.; Hu, M.; Vilavuo, T.; Tolvanen, P.; Cheng, S.; Finni, T. Ventilatory threshold during incremental running can be estimated using EMG shorts. Physiol. Meas. 2012, 33, 603–614. [Google Scholar] [CrossRef]
- Merino, M.; Gómez, I.M.; Molina, A. Envelope filter sequence to delete blinks and overshoots. BioMed. Eng. OnLine. 2015, 14, 48. [Google Scholar] [CrossRef]
- Golparvar, A.J.; Yapici, M.K. Graphene-coated wearable textiles for EOG-based human-computer interaction. In Proceedings of the 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Las Vegas, NV, USA, 4–7 March 2018. [Google Scholar]
- Bulling, A.; Roggen, D.; Tröster, G. It’s in your eyes: Towards context-awareness and mobile HCI using wearable EOG goggles. In Proceedings of the 10th International Conference on Ubiquitous Computing, Seoul, Korea, 21–24 September 2008. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, H.; Park, K.S.J.S. A novel wearable forehead eog measurement system for human computer interfaces. Sensors 2017, 17, 1485. [Google Scholar] [CrossRef]
- Guo, X.; Pei, W.; Wang, Y.; Gong, Q.; Zhang, H.; Xing, X.; Xie, Y.; Gui, Q.; Chen, H. A Self-Wetting Paper Electrode for Ubiquitous Bio-Potential Monitoring. IEEE Sens. J. 2017, 17, 2654–2661. [Google Scholar] [CrossRef]
- Ameri, S.K.; Kim, M.; Kuang, I.A.; Perera, W.K.; Alshiekh, M.; Jeong, H.; Topcu, U.; Akinwande, D.; Lu, N. Imperceptible electrooculography graphene sensor system for human–robot interface. Nature 2018, 2, 19. [Google Scholar] [CrossRef]






| Biopotential Signal | Manufacturing Technique | Conductive Material | System Integration | Electrode Location | |
|---|---|---|---|---|---|
| [22] | ECG | Electroless plating | Silver nanoparticles | Smart garment | Lead 1 and 2 |
| [24] | ECG | Sputtering, electroless plating, knitting, and embroidering | Cu, Ni, stainless steel filament, nylon fabric | T-shirt | Chest |
| [26,74] | ECG | Graphene-coated textile | Graphene | Wristband | Left and right arms |
| [27] | ECG | Printing | PEDOT:PSS | Kimono | Chest and wrists |
| [28] | ECG | Weaving and knitting | Silver yarns | Chest band | Chest |
| [29] | ECG | Knitting | Silver, stainless steel yarn, copper filaments | Garment band | Lead 1 |
| [31] | ECG | Knitting, embroidering, and weaving | Silver | Elastic belt | Chest |
| [41] | ECG | Knitting | Stainless steel | Belt | Chest |
| [57] | ECG | PVD | Ag/Ti-PET yarn | Belt | Chest |
| [63] | ECG | Conductive thread | Silver, PEDOT: PSS | Bras | Lead 1 to 3 |
| [80] | ECG | Printing | Silver | Chest band | Chest |
| [85] | ECG | Ink-jet printing | Graphene | T-shirt | Finger |
| [105] | ECG | Commercial textile | Silver | T-shirt | Lead 1 to 3 |
| [106] | ECG | Screen printing | Silver paste | Band-Aid | Chest |
| [107] | ECG | Knitting | Silver coated yarns | Swimsuit | Chest |
| [108] | ECG | Commercial textile | Silver and gold | Smart jacket | Chest |
| [109] | ECG | Knitting and woven | Stainless steel | Baby suit | Back |
| [110] | ECG | Knitting | Stainless steel threads | T-shirt | RA, LA, LL, RL, V1–V6 |
| [111] | EEG | Seamless knitting | Ag/AgCl-coated thread | Headband | Forehead |
| [112] | EEG | Screen printing | Carbon-loaded rubber | Headband | Forehead |
| [113] | EEG | Screen printing | Ag-particle/fluoropolymer composite ink | Stand-alone | Behind the ears |
| [114] | EMG | Knitting | Silver-plated yarn | Band | Flexor carpi ulnaris |
| [115] | EMG | Knitting | Silver plated nylon | Shirt | Biceps and triceps |
| [116] | EMG | Knitting | Stainless Steel | Sleeve | Upper arm and forearm |
| [117] | EMG | Knitting | Silver Fabric | Hand exoskeleton | Interossei muscles |
| [118] | EMG | Weaving | Silver-plated copper yarn | Eyeglasses | Temporalis muscle |
| [80] | EOG | Screen and stencil printing | Silver Paste | Headband | Forehead |
| [119] | EOG | Coating | Silver | Headband | Forehead |
| [120] | EOG | Commercial textile | Silver/polyamide (20%/80%) compound | Eye mask | Forehead and around eyes |
| [121] | EOG | Dip coating | Graphene | Headband | Forehead |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. https://doi.org/10.3390/electronics8050479
Acar G, Ozturk O, Golparvar AJ, Elboshra TA, Böhringer K, Yapici MK. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics. 2019; 8(5):479. https://doi.org/10.3390/electronics8050479
Chicago/Turabian StyleAcar, Gizem, Ozberk Ozturk, Ata Jedari Golparvar, Tamador Alkhidir Elboshra, Karl Böhringer, and Murat Kaya Yapici. 2019. "Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review" Electronics 8, no. 5: 479. https://doi.org/10.3390/electronics8050479