Physicochemical Properties and the Gelation Process of Supramolecular Hydrogels: A Review
Abstract
:1. Introduction
2. The Structure of Supramolecular Hydrogels
2.1. Design Strategy of Hydrogels: Mechanism of Gelation
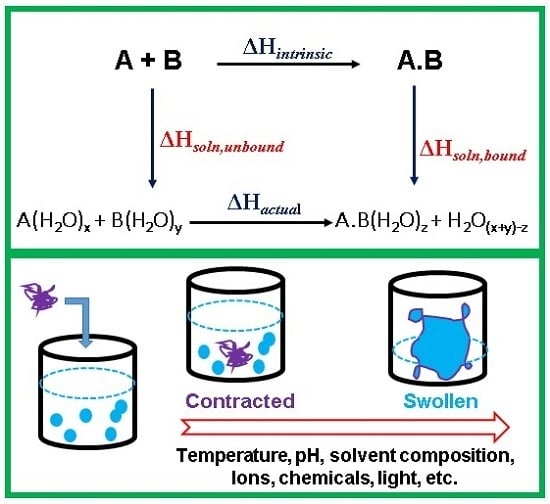
2.2. Volume Phase Transition (VPT) in Polymer Gels
2.3. Classification of Polysaccharide-Based Gels
2.3.1. Single Component Homo-Polymer Gels
2.3.2. Two Component Co-Polymer Gels
2.3.3. Multi-Polymer Inter-Penetrating Networks (IPNs)
2.4. Solvent–Gelator Interactions
2.5. Characterization of Hydrogels
2.5.1. Spectroscopy Techniques
2.5.2. Rheology
2.5.3. Diffraction Techniques
2.5.4. Microscopy Methods
2.5.5. Modeling
2.6. Improving the Stability and Performance of Hydrogels
2.6.1. Use of Polymer Inclusion Complexes (PIC)
2.6.2. Use of Host–Guest Macromers (HGMs)
2.6.3. Use of Amphiphiles
2.6.4. Use of Hybrid Hydrogels and Nano-Fillers
2.7. Modulating the Viscosity: Influence on Host–Guest Complexation
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3D | Three dimensional |
| Ada or AD | Adamantane |
| CD | Cyclodextrin |
| DSC | Differential scanning calorimetry |
| FT-IR | Fourier transform infra-red |
| HGM | Host–guest macromer |
| HLB | Hydrophile lipophile balance |
| ICD | Induced circular dichroism |
| IPN | Interpenetrating network |
| LMW | Low molecular weight |
| NMR | Nuclear magnetic resonance |
| NP | Nanoparticle |
| PEG | Polyethylene glycol |
| PIC | Polymer inclusion network |
| PPR | Polypseudorotaxane |
| SANS | Small-angle neutron scattering |
| SAXS | Small-angle X-ray scattering |
| SEM | Scanning electron microscopy |
| TEM | Total emission microscopy |
| STM | Scanning Tunneling Microscopy |
| UV/Vis | Ultra-violet visible |
| XRD | X-ray diffraction |
References
- Polymer Gels and Networks; Osada, Y.; Khokhlov, A.R. (Eds.) Marcel Dekker, Inc.: New York, NY, USA, 2002.
- Morimoto, N.; Winnik, F.M.; Akiyoshi, K. Botryoidal Assembly of Cholesteryl–Pullulan/Poly(N-isopropylacrylamide) Nanogels. Langmuir 2007, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- De Las Heras Alarcon, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, G.E.; Carrion, B.; Coleman, R.M.; Ostrowski, A.D. Photoresponsive Polysaccharide-Based Hydrogels with Tunable Mechanical Properties for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 14423–14429. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.; Gao, C.; Xu, X.; Bai, X.; Duan, H.; Gao, N.; Feng, C.; Xiong, Y.; Liu, M. Injectable and Self-Healing Carbohydrate-Based Hydrogel for Cell Encapsulation. ACS Appl. Mater. Interfaces 2015, 7, 13029–13037. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Shetye, S.P.; Godbole, A.; Bhilegaokar, S.; Gajare, P. Hydrogels: Introduction, Preparation, Characterization and Applications. Hum. J. 2015, 1, 47–71. [Google Scholar]
- Paulino, A.T.; Belfiore, L.A.; Kubota, L.T.; Muniz, E.C.; Tambourgi, E.B. Efficiency of hydrogels based on natural polysaccharides in the removal of Cd2+ ions from aqueous solutions. Chem. Eng. J. 2011, 168, 68–76. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Fajardo, A.R.; Muniz, E.C.; Tambourgi, E.B. Superabsorbent hydrogel based on modified polysaccharide for removal of Pb2+ and Cu2+ from water with excellent performance. J. Appl. Polym. Sci. 2007, 105, 2903–2909. [Google Scholar] [CrossRef]
- Copello, G.J.; Mebert, A.M.; Raineri, M.; Pesenti, M.P.; Diaz, L.E. Removal of dyes from water using chitosan hydrogel/SiO2 and chitin hydrogel/SiO2 hybrid materials obtained by the sol–gel method. J. Hazard. Mater. 2011, 186, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Udoetok, I.; Wilson, L.; Headley, J. Quaternized Cellulose Hydrogels as Sorbent Materials and Pickering Emulsion Stabilizing Agents. Materials (Basel) 2016, 9, 645. [Google Scholar] [CrossRef]
- Encapsulation Technologies and Delivery Systems for Food Ingrediaent and Nutraceuticals; Garti, N.; McClements, D.J. (Eds.) Woodhead Publishing Ltd.: Cambridge, UK, 2012.
- Parente, M.E.; Ochoa, A.A.; Ares, G.; Russo, F.; Jimenez-Kairuz, A. Bioadhesive Hydrogels for Cosmetic Applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Schaubroeck, D.; Luickx, N.; Dubruel, P.; van Vlierberghe, S.; de Belie, N. pH-Responsive superabsorbent polymers: A pathway to self-healing of mortar. React. Funct. Polym. 2015, 93, 68–76. [Google Scholar] [CrossRef]
- Mignon, A.; Graulus, G.J.; Snoeck, D.; Martins, J.; de Belie, N.; Dubruel, P.; van Vlierberghe, S. pH-Sensitive superabsorbent polymers: A potential candidate material for self-healing concrete. J. Mater. Sci. 2014, 50, 970–979. [Google Scholar] [CrossRef]
- Hyon, S.; Cha, W.; Ikada, Y. Polymer Bulletin 9. Polym. Bull. 1987, 29, 119–126. [Google Scholar]
- Milas, M.; Rinaud, M. Gellan gum, a bacterial gelling polymer. In Novel Macromolecules in Food Systems; Doxastakis, G., Kiosseogluou, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 239–263. [Google Scholar]
- Rinaudo, M. Advances in Characterization of Polysaccharides in Aqueous Solution and Gel State. In Polysaccharides: Structural Diversity and Functional Versitility; Dumitriu, S., Ed.; Marcel Decker: New York, NY, USA, 2004; p. 237. [Google Scholar]
- Rinaudo, M. Gelation of Polysaccharides. J. Intell. Mater. Syst. Struct. 1993, 4, 210–215. [Google Scholar] [CrossRef]
- Saitô, H.; Ohki, T.; Takasuka, N.; Sasaki, T. A 13C-N.M.R.-Spectral study of a gel-forming, branched (1→3)-β-d-glucan, (lentinan) from lentinus edodes, and its acid-degraded fractions. Structure, and dependence of conformation on the molecular weight. Carbohyd. Res. 1977, 58, 293–305. [Google Scholar] [CrossRef]
- Pasqui, D.; de Cagna, M.; Barbucci, R. Polysaccharide-based hydrogels: The key role of water in affecting mechanical properties. Polymers (Basel) 2012, 4, 1517–1534. [Google Scholar] [CrossRef]
- Crescenzi, V.; Paradossi, G.; Desideri, P.; Dentini, M.; Cavalieri, F.; Amici, E.; Lisi, R. New hydrogels based on carbohydrate and on carbohydrate-synthetic polymer networks. Polym. Gels Netw. 1997, 5, 225–239. [Google Scholar] [CrossRef]
- Himmelein, S.; Lewe, V.; Stuart, M.C.A.; Ravoo, B.J. A carbohydrate-based hydrogel containing vesicles as responsive non-covalent cross-linkers. Chem. Sci. 2014, 5, 1054–1058. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, Y.; Taira, T.; Osakada, K. Physical gels based on supramolecular gelators, including host–guest complexes and pseudorotaxanes. J. Mater. Chem. 2011, 21, 930–938. [Google Scholar] [CrossRef]
- Sukul, P.K.; Malik, S. Supramolecular hydrogels of adenine: Morphological, structural and rheological investigations. Soft Matter 2011, 7, 4234–4241. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Kiyonaka, S.; Sugiyasu, K.; Shinkai, S.; Hamachi, I. First thermally responsive supramolecular polymer based on glycosylated amino acid. J. Am. Chem. Soc. 2002, 124, 10954–10955. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Xu, J.; Hu, J.; Zhang, Y.; Liu, S. Synthesis and supramolecular self-assembly of stimuli-responsive water-soluble Janus-type heteroarm star copolymers. Soft Matter 2009, 5, 3932–3939. [Google Scholar] [CrossRef]
- Ren, L.; Liu, T.; Guo, J.; Guo, S.; Wang, X.; Wang, W. A smart pH responsive graphene/polyacrylamide complex via noncovalent interaction. Nanotechnology 2010, 21, 335701–335706. [Google Scholar] [CrossRef] [PubMed]
- Klaikherd, A.; Nagamani, C.; Thayumanavan, S. Multi-Stimuli Sensitive Amphilic Block Copolymer Assemblies. J. Am. Chem. Soc. 2009, 131, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, H.; Zheng, J.; Gan, J.; Wu, K.; Lu, M. Thermoresponsive and self-assembly behaviors of poly(oligo(ethylene glycol) methacrylate) based cyclodextrin cored star polymer and pseudo-graft polymer. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 471, 178–189. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, T.; Chi, C. Thermoresponsive oligo(ethylene glycol)-methacrylate-based polymers and microgels. Soft Matter 2010, 6, 2115–2123. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, M.; Liang, H.; Wu, C. Intermacromolecular complexes due to specific interactions. 13. Formation of micelle-like structure from hydrogen-bonding graft-like complexes in selective solvents. Polymer (Guildf) 2000, 41, 8697–8702. [Google Scholar] [CrossRef]
- Gohy, J.F.; Varshney, S.K.; Jérôme, R. Water-soluble complexes formed by poly(2-vinylpyridinium)-block-poly(ethylene oxide) and poly(sodium methacrylate)-block-poly(ethylene oxide) copolymers. Macromolecules 2001, 34, 3361–3366. [Google Scholar] [CrossRef]
- Kim, J., II; Kim, D.Y.; Kwon, D.Y.; Kang, H.J.; Kim, J.H.; Min, B.H.; Kim, M.S. An injectable biodegradable temperature-responsive gel with an adjustable persistence window. Biomaterials 2012, 33, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.T.; Li, W.; Zhang, X.Q.; Liu, K.; Wu, P.Y.; Zhang, A.F. Thermoresponsive cyclodextrins with switchable inclusion abilities. J. Mater. Chem. 2012, 22, 17424–17428. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Dimmick, R.M.; Wilson, L.D.; Headley, J.V. Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution. Carbohydr. Polym. 2016, 136, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Jejurikar, A.; Lawrie, G.; Martin, D.; Grøndahl, L. A novel strategy for preparing mechanically robust ionically cross-linked alginate hydrogels. Biomed. Mater. 2011, 6, 025010–025021. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.R.; Soppimath, K.S.; Aminabhavi, T.M.; Rudzinski, W.E. In-vitro release kinetics of cefadroxil-loaded sodium alginate interpenetrating network beads. Eur. J. Pharm. Biopharm. 2001, 51, 127–133. [Google Scholar] [CrossRef]
- Seoud, M.A.; Maachi, R. Biodegradation of Naphthalene by Free and Alginate Entrapped Pseudomonas sp. Z. Naturforsch. C 2003, 58, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Lehnen, R.; Schmitt, U.; Saake, B. Effect of oat spelt and beech xylan on the gelling properties of kappa-carrageenan hydrogels. Carbohydr. Polym. 2011, 85, 529–540. [Google Scholar] [CrossRef]
- Holme, K.R.; Hall, L.D. Chitosan Derivatives Bearing C10-Alkyl Glycoside Branches: A Temperature-Induced Gelling Polysaccharide. Macromolecules 1991, 24, 3828–3833. [Google Scholar] [CrossRef]
- Djabourov, M.; Leblond, J.; Papon, P. Gelation of acqueous gelatin solutions. II. Rheology of the sol–gel transition. J. Phys. Fr. 1988, 49, 333–343. [Google Scholar] [CrossRef]
- Wei, K.; Zhu, M.; Sun, Y.; Xu, J.; Feng, Q.; Lin, S.; Wu, T.; Xu, J.; Tian, F.; Xia, J.; et al. Robust Biopolymeric Supramolecular “Host-Guest Macromer” Hydrogels Reinforced by in Situ Formed Multivalent Nanoclusters for Cartilage Regeneration. Macromolecules 2016, 49, 866–875. [Google Scholar] [CrossRef]
- Yoshida, H.; Takahashi, M. Structural-Change of Gellan Hydrogel Induced by Annealing. Food Hydrocoll. 1993, 7, 387–395. [Google Scholar] [CrossRef]
- Hosseinzadeh, H. Full-Polysaccharide Superabsorbent Hydrogels Based on Carrageenan and Sodium Alginate. Middle-East J. Sci. Res. 2012, 12, 1521–1527. [Google Scholar]
- Jung, J.H.; John, G.; Masuda, M.; Yoshida, K.; Shinkai, S.; Shimizu, T. Self-assembly of a sugar-based gelator in water: Its remarkable diversity in gelation ability and aggregate structure. Langmuir 2001, 17, 7229–7232. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, B.; Ma, P.X. Injectable electroactive hydrogels formed via host-guest interactions. ACS Macro Lett. 2014, 3, 1145–1150. [Google Scholar] [CrossRef]
- Yu, J.; Ha, W.; Sun, J.; Shi, Y. Supramolecular Hybrid Hydrogel Based on Host–Guest Interaction and Its Application in Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 19544–19551. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chen, G.; Liu, X.; Chen, W.; Chen, F.; Jiang, M. Photoresponsive pseudopolyrotaxane hydrogels based on competition of host-guest interactions. Angew. Chemie Int. Ed. 2010, 49, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Amiel, C.; Sebille, B. New Associating polymer systems Involving Water Soluble β-Cyclodextrin Polymers. J. Incl. Phenom. 1996, 25, 61–67. [Google Scholar] [CrossRef]
- Koopmans, C.; Ritter, H. Formation of physical hydrogels via host-guest interactions of β-cyclodextrin polymers and copolymers bearing adamantyl groups. Macromolecules 2008, 41, 7416–7422. [Google Scholar] [CrossRef]
- Annaka, M.; Ogata, Y.; Nakahira, T. Swelling Behavior of Covalently Cross-Linked Gellan Gels. J. Phys. Chem. B 2000, 104, 6755–6760. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Chang, P.R.; Li, J.; Chen, Y.; Wang, D.; Yu, J.; Chen, J. Structure and properties of polysaccharide nanocrystal-doped supramolecular hydrogels based on cyclodextrin inclusion. Polymer 2010, 51, 4398–4407. [Google Scholar] [CrossRef]
- Choi, H.S.; Kontani, K.; Huh, K.M.; Sasaki, S.; Ooya, T.; Lee, W.K.; Yui, N. Rapid Induction of Thermoreversible Hydrogel Formation Based on Poly(propylene glycol)-Grafted Dextran Inclusion Complexes. Macromol. Biosci. 2002, 2, 298–303. [Google Scholar] [CrossRef]
- Huh, K.M.; Ooya, T.; Lee, W.K.; Sasaki, S.; Kwon, I.C.; Jeong, S.Y.; Yui, N. Supramolecular-structured hydrogels showing a reversible phase transition by inclusion complexation between poly(ethylene glycol) grafted dextran and α-cyclodextrin. Macromolecules 2001, 34, 8657–8662. [Google Scholar] [CrossRef]
- Zhao, S.; Lee, J.; Xu, W. Supramolecular hydrogels formed from biodegradable ternary COS-g-PCL-b-MPEG copolymer with α-cyclodextrin and their drug release. Carbohydr. Res. 2009, 344, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- Maity, G.C. Supramolecular Hydrogels. J. Phys. Sci. 2008, 12, 173–186. [Google Scholar]
- Guo, M.; Jiang, M.; Pispas, S.; Yu, W.; Zhou, C. Supramolecular Hydrogels Made of End-Functionalized Low-Molecular-Weight PEG and α-Cyclodextrin and Their Hybridization with SiO2 Nanoparticles through Host–Guest Interaction. Macromolecules 2008, 41, 9744–9749. [Google Scholar] [CrossRef]
- Tanaka, F. Thermoreversible gelation strongly coupled to coil-to-helix transition of polymers. Colloids Surf. B Biointerfaces 2004, 38, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Phase transitions of gels. Nippon Gomu Kyokaishi 1991, 64, 219–231. [Google Scholar] [CrossRef]
- Viebke, C.; Piculell, L.; Nilssont, S. On the Mechanism of Gelation of Helix-Forming Biopolymers. Macromolecules 1994, 27, 4160–4166. [Google Scholar] [CrossRef]
- Shukla, P. Thermodynamics and kinetics of gelation in the poly(γ-benzyl α,l-glutamate)—Benzyl alcohol system. Polymer (Guildf) 1992, 33, 365–372. [Google Scholar] [CrossRef]
- Moris, E.R.; Rees, D.A.; Robinson, G. Cation-specific aggregation of carrageenan helices: Domain model of polymer gel structure. J. Mol. Biol. 1980, 138, 349–362. [Google Scholar] [CrossRef]
- Dusek, K. My fifty years with polymer gels and networks and beyond. Polym. Bull. 2007, 58, 321–338. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T. Volume phase transition and related phenomena of polymer gels. Adv. Polym. Sci. 1993, 109, 1–62. [Google Scholar]
- Tanaka, T.; Filmore, D.; Sun, S.T.; Nishio, I.; Swislow, G.; Shah, A. Phase Transition in Ionic Gels. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar] [CrossRef]
- Das, N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Aminabhavi, T.M.; Deshmukh, A.M. Polysaccharide-Based Hydrogels as Biomaterials. In Polymeric Hydrogels as Smart Biomaterials; Kalia, S., Ed.; Springer: Basel, Switzerland, 2016; pp. 45–71. [Google Scholar]
- Deng, W.; Yamaguchi, H.; Takashima, Y.; Harada, A. A chemical-responsive supramolecular hydrogel from modified cyclodextrins. Angew. Chemie Int. Ed. 2007, 46, 5144–5147. [Google Scholar] [CrossRef] [PubMed]
- Van de Manakker, F.; van der Pot, M.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Self-Assembling Hydrogels Based on β-Cyclodextrin/Cholesterol Inclusion Complexes. Macromolecules 2008, 41, 1766–1773. [Google Scholar] [CrossRef]
- Kalichevsky, M.T.; Orford, P.D.; Ring, S.G. The incompatibility of concentrated aqueous solutions of dextran and amylose and its effect on amylose gelation. Carbohydr. Polym. 1986, 6, 145–154. [Google Scholar] [CrossRef]
- Shivashankar, M.; Mandal, B.K. A review on interpenetrating polymer network. Int. J. Pharm. Pharm. Sci. 2012, 4, 1–7. [Google Scholar]
- Myung, D.; Waters, D.J.; Wiseman, M.E. Al Progress in the development of interpenetrating network hydrogels. Polym. Adv. Technol. 2008, 19, 4109. [Google Scholar] [CrossRef] [PubMed]
- Al-Kahtani, A.A.; Sherigara, B.S. Controlled release of theophylline through semi-interpenetrating network microspheres of chitosan-(dextran-g-acrylamide). J. Mater. Sci. Mater. Med. 2009, 20, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Blokzijl, B.W.; Engberts, J.B.F.N. Hydrophobic Effects. Opinions and Facts. Angew. Chem. Int. Ed. Engl. 1993, 32, 1545–1579. [Google Scholar] [CrossRef]
- Edwards, W.; Lagadec, C.A.; Smith, D.K. Solvent-gelator interactions-using empirical solvent parameters to better understand the self-assembly of gel-phase materials. Soft Matter 2011, 7, 110–117. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvents Effects in Organic Chemistry, 4th ed.; Wiley-VCH Verlag GMbH&Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, .pi.*, alpha., and beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Lagadec, C.; Smith, D.K. Synthetically accessible, tunable, low-molecular-weight oligopeptide organogelators. Chem. Commun. 2011, 47, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Smith, D.K. Solvent effects on supramolecular gel-phase materials: Two-component dendritic gel. Langmuir 2004, 20, 10851–10857. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Characterization of Sol-Gel Science and Technology: Processing, Characterization and Applications; Almeida, R.M. (Ed.) Kuwer Academic Publishers: Boston, MA, USA, 2005.
- Birchall, L.S.; Roy, S.; Jayawarna, V.; Hughes, M.; Irvine, E.; Okorogheye, G.T.; Saudi, N.; de Santis, E.; Tuttle, T.; Edwards, A.A.; et al. Exploiting CH-π interactions in supramolecular hydrogels of aromatic carbohydrate amphiphiles. Chem. Sci. 2011, 2, 1349. [Google Scholar] [CrossRef] [Green Version]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2009. [Google Scholar]
- Ma, D.; Xie, X.; Zhang, L.-M. Effect of Molecular Weight and Temperature on Physical Aging of Thin Glassy Poly(2,6-dimethyl-1,4-phenylene oxide) Films. J. Polym. Sci. B Polym. Phys. 2007, 45, 1390–1398. [Google Scholar]
- Decho, A.W. Imaging an alginate polymer gel matrix using atomic force microscopy. Carbohydr. Res. 1999, 315, 330–333. [Google Scholar] [CrossRef]
- Yin, L.; Fei, L.; Cui, F.; Tang, C.; Yin, C. Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials 2007, 28, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Tinland, B.; Sadron, I.C.; Pasteur, C.L. Pore size of agarose gels by atomic force microscopy. Electrophoresis 1997, 18, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, L.M. Fabrication and modulation of magnetically supramolecular hydrogels. J. Phys. Chem. B 2008, 112, 6315–6321. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Ren, C.; Wang, J.; Tan, M.; Shen, J.; Yang, Z.; Wang, P.G.; Wang, L. A saccharide-based supramolecular hydrogel for cell culture. Carbohydr. Res. 2011, 346, 1013–1017. [Google Scholar] [CrossRef] [PubMed]







| Origin | Gelator/Precursor | Responsive Feature | References |
|---|---|---|---|
| Plant cell walls, wood, seeds, & roots | Pectins, cellulose, galacto-/gluco-mannans | Chemical species (arsenic), pH, & temperature | [21,25,26,41] |
| Seaweeds | Carrageenans, alginates, agar | Light, & temperature | [5,42,43,44,45] |
| Animals, organisms, bacteria | Hyaluronan, chitosan, chondroitins, xanthan, succinoglycan, gelatin, gellan | Temperature, & pH | [19,46,47,48,49,50] |
| Sugars | Cyclodextrins, galactose, glucose | Redox, light, temperature, & chemical species | [25,51,52,53,54] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoyo, A.H.; Wilson, L.D. Physicochemical Properties and the Gelation Process of Supramolecular Hydrogels: A Review. Gels 2017, 3, 1. https://doi.org/10.3390/gels3010001
Karoyo AH, Wilson LD. Physicochemical Properties and the Gelation Process of Supramolecular Hydrogels: A Review. Gels. 2017; 3(1):1. https://doi.org/10.3390/gels3010001
Chicago/Turabian StyleKaroyo, Abdalla H., and Lee D. Wilson. 2017. "Physicochemical Properties and the Gelation Process of Supramolecular Hydrogels: A Review" Gels 3, no. 1: 1. https://doi.org/10.3390/gels3010001