A Polymer Visualization System with Accurate Heating and Cooling Control and High-Speed Imaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. System Development




2.2. Measurement of Isothermal Crystallization with High-Pressure Differential Scanning Calorimetry



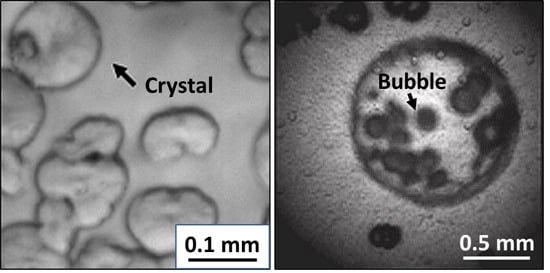
2.3. In Situ Observation of Crystal Formation and Foaming Processes
| Material | Tiso [°C] | Material | Tiso [°C] |
|---|---|---|---|
| Linear PP | 124 | PP-ethylene copolymer | 112 |
| 127 | 115 | ||
| 130 | 118 | ||
| 133 | 121 | ||
| 136 | 124 | ||
| 139 | 127 | ||
| 130 | |||




3. Experimental Section
3.1. Experimental Materials
3.2. Measurement of Isothermal Crystallization with High-Pressure Differential Scanning Calorimetry
3.3. In Situ Observation of Crystal Formation and Foaming Processes

4. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| A | pre-exponent parameter for the cell nucleation rate equation, #/cm3-s |
| F | ratio of a nucleated bubble’s volume to a spherical bubble’s volume with the same radius, dimensionless |
| kb | Boltzmann’s Constant, m2-kg/s2-K |
| J | cell nucleation rate, #/cm3-s |
| Pbub,cr | bubble pressure at the critical radius, Pa |
| ΔPlocal | local system pressure around a bubble, Pa |
| Psat | saturation pressure, Pa |
| Psys | system pressure, Pa |
| −dPsys/dt|avg | average pressure drop rate, Pa/s |
| Rcr | critical radius, cm |
| Tc | crystallization temperature, °C |
| Tiso | isothermal temperature, °C |
| Tm | melting temperature, °C |
| Tsys | system temperature, °C |
| W | free energy barrier for cell nucleation, J |
| θc | contact angle, ° |
| γlg | surface tension at the polymer/gas interface, N/m |
Conflicts of Interest
References
- Wong, A.; Guo, Y.; Park, C.B.; Zhou, N.Q. Isothermal crystallization-induced foaming of polypropylene under high pressure carbon dioxide. In Proceedings of the Society of Plastics Engineers Annual Technical Conference, Orlando, FL, USA, 2–4 April 2012; Volume 3, pp. 2443–2449.
- Nofar, M.; Tabatabaei, A.; Ameli, A.; Park, C.B. Comparison of melting and crystallization behaviors of polylactide under high-pressure CO2, N2, and He. Polymer 2013, 54, 6471–6478. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Park, C.B. Effects of nano-/micro-sized additives on the crystallization behaviors of PLA and PLA/CO2 mixtures. Polymer 2013, 54, 2382–2391. [Google Scholar] [CrossRef]
- Nofar, M.; Zhu, W.; Park, C.B. Effect of dissolved CO2 on the crystallization behavior of linear and branched PLA. Polymer 2012, 53, 3341–3353. [Google Scholar]
- Nofar, M.; Ameli, A.; Park, C.B. The thermal behavior of polylactide with different d-lactide content in the presence of dissolved CO2. Macromol. Mater. Eng. 2014, 299, 1232–1239. [Google Scholar] [CrossRef]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene. II: The morphology of the γ-form crystallized at 200 MPa. Polymer 1997, 38, 5725–5733. [Google Scholar] [CrossRef]
- Zhai, W.; Ko, Y.; Zhu, W.; Wong, A.; Park, C.B. A study of the crystallization, melting, and foaming behaviors of polylactic acid in compressed CO2. Int. J. Mol. Sci. 2009, 10, 5381–5397. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Kitano, D.; Ohshima, M. Effect of growing crystalline phase on bubble nucleation in poly(L-lactide)/CO2 batch foaming. Ind. Eng. Chem. Res. 2011, 50, 3247–3252. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D. Crystallinity development in cellular poly(lactic acid) in the presence of supercritical carbon dioxide. J. Appl. Polym. Sci. 2009, 113, 2920–2932. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D. Rheology and extrusion foaming of chain-branched poly(lactic acid). Polym. Eng. Sci. 2010, 50, 629–642. [Google Scholar] [CrossRef]
- Zhai, W.; Kuboki, T.; Wang, L.; Park, C.B.; Lee, E.K.; Naguib, H.E. Cell structure evolution and the crystallization behavior of polypropylene/clay nanocomposites foams blown in continuous extrusion. Ind. Eng. Chem. Res. 2010, 49, 9834–9845. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Turng, L.S.; Zhao, L.; Liu, T.; Yuan, W.K. Investigation of crystallization behavior of solid and microcellular injection molded polypropylene/nano-calcium carbonate composites using carbon dioxide as a blowing agent. J. Cell Plast. 2013, 49, 459–475. [Google Scholar] [CrossRef]
- Wong, A.; Guo, Y.; Park, C.B. Fundamental mechanisms of cell nucleation in polypropylene foaming with supercritical carbon dioxide-effects of extensional stresses and crystals. J. Supercrit. Fluids 2013, 79, 142–151. [Google Scholar] [CrossRef]
- Liao, R.; Yu, W.; Zhou, C. Rheological control in foaming polymeric materials: II. Semi-crystalline polymers. Polymer 2010, 51, 6334–6345. [Google Scholar] [CrossRef]
- Keshtkar, M.; Nofar, M.; Park, C.B.; Carreau, P.J. Extruded PLA/clay nanocomposite foams blown with supercritical CO2. Polymer 2014, 55, 4077–4090. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Reignier, J.; Tatibouët, J.; Gendron, R. Batch foaming of poly(ε-caprolactone) using carbon dioxide: Impact of crystallization on cell nucleation as probed by ultrasonic measurements. Polymer 2006, 47, 5012–5024. [Google Scholar] [CrossRef]
- Bao, J.B.; Liu, T.; Zhao, L.; Barth, D.; Hu, G.H. Supercritical carbon dioxide induced foaming of highly oriented isotactic polypropylene. Ind. Eng. Chem. Res. 2011, 50, 13387–13395. [Google Scholar] [CrossRef]
- Doroudiani, S.; Park, C.B.; Kortschot, M.T. Effect of the crystallinity and morphology on the microcellular foam structure of semicrystalline polymers. Polym. Eng. Sci. 1996, 36, 2645–2662. [Google Scholar] [CrossRef]
- Doroudiani, S.; Park, C.B.; Kortschot, M.T. Processing and characterization of microcellular foamed high-density polyethylene/isotactic polypropylene blends. Polym. Eng. Sci. 1998, 38, 1205–1215. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Zhang, H.; Park, C.B. Continuous processing of low-density, microcellular poly(lactic acid) foams with controlled cell morphology and crystallinity. Chem. Eng. Sci. 2012, 75, 390–399. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Guan, Y.; Wei, D.; Zheng, A. Investigation of extensional rheological behaviors of polypropylene for foaming. J. Cell Plast. 2013, 49, 317–334. [Google Scholar] [CrossRef]
- Guo, P.; Liu, Y.; Xu, Y.; Lu, M.; Zhang, S.; Liu, T. Effects of saturation temperature/pressure on melting behavior and cell structure of expanded polypropylene bead. J. Cell Plast. 2014, 50, 321–335. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, J.; Zhai, W.; Su, S. Solid state foaming of poly(lactic acid) blown with compressed CO2: Influences of long chain branching and induced crystallization on foam expansion and cell morphology. Ind. Eng. Chem. Res. 2013, 52, 13411–13421. [Google Scholar] [CrossRef]
- Guo, Y.; Hossieny, N.; Chu, R.K.M.; Park, C.B.; Zhou, N. Critical processing parameters for foamed bead manufacturing in a lab-scale autoclave system. Chem. Eng. J. 2013, 214, 180–188. [Google Scholar] [CrossRef]
- Naguib, H.E.; Park, C.B.; Reichelt, N. Fundamental foaming mechanisms governing the volume expansion of extruded polypropylene foams. J. Appl. Polym. Sci. 2004, 91, 2661–2668. [Google Scholar] [CrossRef]
- Spitael, P.; Macosko, C.W. Strain hardening in polypropylenes and its role in extrusion foaming. Polym. Eng. Sci. 2004, 44, 2090–2100. [Google Scholar] [CrossRef]
- Stange, J.; Münstedt, H. Rheological properties and foaming behavior of polypropylenes with different molecular structures. J. Rheol. 2006, 50, 907–923. [Google Scholar] [CrossRef]
- Sharudin, R.W. B.; Ohshima, M. CO2-induced mechanical reinforcement of polyolefin-based nanocellular foams. Macromol. Mater. Eng. 2011, 296, 1046–1054. [Google Scholar] [CrossRef]
- Oda, T.; Saito, H. Exclusion effect of carbon dioxide on the crystallization of polypropylene. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1565–1572. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.M.; Xie, B.H.; Feng, J.M.; Shi, W.; Yang, M.B. Stress-induced crystallization of biaxially oriented polypropylene. J. Appl. Polym. Sci. 2003, 89, 686–690. [Google Scholar] [CrossRef]
- Han, C.D.; Villamizar, C.A. Studies of structural foam processing I. The rheology of foam extrusion. Polym. Eng. Sci. 1978, 18, 687–698. [Google Scholar] [CrossRef]
- Villamizar, C.A.; Dae Han, C. Studies of structural foam processing II. Bubble dynamics in foam injection molding. Polym. Eng. Sci. 1978, 18, 699–710. [Google Scholar] [CrossRef]
- Taki, K.; Nakayama, T.; Yatsuzuka, T.; Ohshima, M. Visual observations of batch and continuous foaming processes. J. Cell Plast. 2003, 39, 155–169. [Google Scholar] [CrossRef]
- Tsujimura, I.; Murayama, T.; Zenki, T.; Ikeda, J.; Ishida, M.; Masuoka, H. A study of bubble nucleation in foam extrusion die. Seikei Kako 1999, 11, 937. [Google Scholar] [CrossRef]
- Zhang, Q.; Xanthos, M.; Dey, S.K. Parameters affecting the in-line measurement of gas solubility in thermoplastic melts during foam extrusion. J. Cell Plast. 2001, 37, 284–292. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ohshima, M. Visual observation and numerical studies of polymer foaming behavior of polypropylene/carbon dioxide system in a core-back injection molding process. Polym. Eng. Sci. 2011, 51, 1617–1625. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Behravesh, A.H.; Mohammad Rezavand, S.A.; Pashaei, A. Visualization of bubble dynamics in foam injection molding. J. Appl. Polym. Sci. 2010, 116, 3346–3355. [Google Scholar]
- Mahmoodi, M.; Behravesh, A.H.; Rezavand, S.A.M.; Golzar, M. Theoretical and visual study of bubble dynamics in foam injection molding. Polym. Eng. Sci. 2010, 50, 561–569. [Google Scholar] [CrossRef]
- Han, J.H.; Han, C.D. Study of bubble nucleation in a mixture of molten polymer and volatile liquid in a shear flow field. Polym. Eng. Sci. 1988, 28, 1616–1627. [Google Scholar] [CrossRef]
- Han, J.H.; Han, C.D. Bubble nucleation in polymeric liquids. II. Theoretical considerations. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 743–761. [Google Scholar] [CrossRef]
- Han, J.H.; Han, C.D. Bubble nucleation in polymeric liquids. I. Bubble nucleation in concentrated polymer solutions. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 711–741. [Google Scholar] [CrossRef]
- Tatibouët, J.; Gendron, R. A study of strain-induced nucleation in thermoplastic foam extrusion. J. Cell Plast. 2004, 40, 27–44. [Google Scholar] [CrossRef]
- Sahnoune, A.; Tatibouët, J.; Gendron, R.; Hamel, A.; PichÉ, L. Application of ultrasonic sensors in the study of physical foaming agents for foam extrusion. J. Cell Plast. 2001, 37, 429–454. [Google Scholar] [CrossRef]
- Otake, K.; Sugeta, T.; Yoda, S.; Takebayashi, Y. Dynamics of microcellular structure formation. In Proceedings of the Japanese Society of Polymer Processing Polymer Processing Symposia 2000, Hiroshima, Japan, April 2000; pp. 219–220.
- Guo, Q.; Wang, J.; Park, C.B.; Ohshima, M. A microcellular foaming simulation system with a high pressure-drop rate. Ind. Eng. Chem. Res. 2006, 45, 6153–6161. [Google Scholar] [CrossRef]
- Salejova, G.; Kosek, J. Dynamics of foaming of polystyrene particles. Macromol. Symp. 2006, 243, 233–246. [Google Scholar] [CrossRef]
- Wong, A.; Chu, R.K.M.; Leung, S.N.; Park, C.B.; Zong, J.H. A batch foaming visualization system with extensional stress-inducing ability. Chem. Eng. Sci. 2011, 66, 55–63. [Google Scholar] [CrossRef]
- Wong, A.; Park, C.B. A visualization system for observing plastic foaming processes under shear stress. Polym. Test. 2012, 31, 417–424. [Google Scholar] [CrossRef]
- Mackley, M.R.; Marshall, R.T.J.; Smeulders, J.B.A.F. The multipass rheometer. J. Rheol. 1995, 39, 1293–1309. [Google Scholar] [CrossRef]
- Mackley, M.R.; Spitteler, P.H.J. Experimental observations on the pressure-dependent polymer melt rheology of linear low density polyethylene, using a multi-pass rheometer. Rheol. Acta 1996, 35, 202–209. [Google Scholar] [CrossRef]
- Suleimenov, O.M. Simple, compact, flow-through, high temperature high pressure cell for UV-Vis spectrophotometry. Rev. Sci. Instrum. 2004, 75, 3363–3364. [Google Scholar] [CrossRef]
- Bridgman, P.W. The Physics of High Pressure; Dover Publications Inc.: New York, NY, USA, 1970. [Google Scholar]
- Choi, J.B.; Chung, M.J.; Yoon, J.S. Formation of double melting peak of poly(propylene-co-ethylene-co-1-butene) during the preexpansion process for production of expanded polypropylene. Ind. Eng. Chem. Res. 2005, 44, 2776–2780. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P.; Minakov, A.A.; Adamovsky, S.; Schick, C.; Hashimoto, T.; Saijo, K. Crystallization of polypropylene at various cooling rates. Mater. Sci. Eng. A 2005, 413–414, 442–446. [Google Scholar]
- Wasiak, A.; Sajkiewicz, P.; Woźniak, A. Effects of cooling rate on crystallinity of i-polypropylene and polyethylene terephthalate crystallized in nonisothermal conditions. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2821–2827. [Google Scholar] [CrossRef]
- Zweifel, H.; Maier, R.D.; Schiller, M. Plastics Additives Handbook; Hanser: Munich, Germany, 2009. [Google Scholar]
- Norton, D.R.; Keller, A. The spherulitic and lamellar morphology of melt-crystallized isotactic polypropylene. Polymer 1985, 26, 704–716. [Google Scholar] [CrossRef]
- Koga, Y.; Saito, H. Porous structure of crystalline polymers by exclusion effect of carbon dioxide. Polymer 2006, 47, 7564–7571. [Google Scholar] [CrossRef]
- Wong, A.; Park, C.B. The effects of extensional stresses on the foamability of polystyrene-talc composites blown with carbon dioxide. Chem. Eng. Sci. 2012, 75, 49–62. [Google Scholar] [CrossRef]
- Gibbs, J.W. The Scientific Papers of J. Willard Gibbs Volume 1; Dover Publications Inc.: New York, NY, USA, 1961. [Google Scholar]
- Blander, M.; Katz, J.L. Bubble nucleation in liquids. AlChE J. 1975, 21, 833–848. [Google Scholar] [CrossRef]
- Leung, S.N.; Wong, A.; Wang, C.; Park, C.B. Mechanism of extensional stress-induced cell formation in polymeric foaming processes with the presence of nucleating agents. J. Supercrit. Fluids 2012, 63, 187–198. [Google Scholar] [CrossRef]
- Guo, Q. Visualization of Polymer Foaming Using a Batch Foaming Simulation System with a High Pressure-Drop Rate. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2007. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, A.; Guo, Y.; Park, C.B.; Zhou, N.Q. A Polymer Visualization System with Accurate Heating and Cooling Control and High-Speed Imaging. Int. J. Mol. Sci. 2015, 16, 9196-9216. https://doi.org/10.3390/ijms16059196
Wong A, Guo Y, Park CB, Zhou NQ. A Polymer Visualization System with Accurate Heating and Cooling Control and High-Speed Imaging. International Journal of Molecular Sciences. 2015; 16(5):9196-9216. https://doi.org/10.3390/ijms16059196
Chicago/Turabian StyleWong, Anson, Yanting Guo, Chul B. Park, and Nan Q. Zhou. 2015. "A Polymer Visualization System with Accurate Heating and Cooling Control and High-Speed Imaging" International Journal of Molecular Sciences 16, no. 5: 9196-9216. https://doi.org/10.3390/ijms16059196