Preparation of Silk Sericin/Lignin Blend Beads for the Removal of Hexavalent Chromium Ions
Abstract
:1. Introduction
2. Results and Discussion
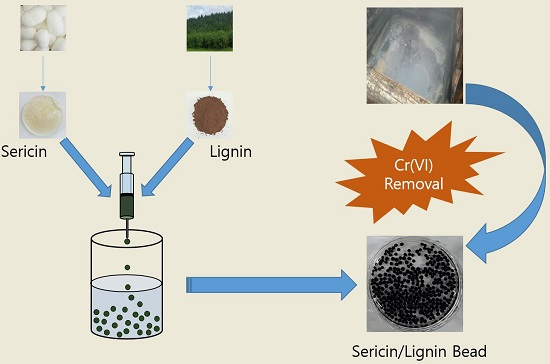
2.1. Preparation of Silk Sericin/Kraft Lignin Blend Beads
2.2. Cr(VI) Adsorption onto SS/KL Beads
2.3. Cr(VI) Adsorption Behavior
2.3.1. Effect of pH on the Cr(VI) Removal Process
2.3.2. Effect of Initial Concentration and Adsorption Isotherms
2.3.3. Adsorption Kinetics and Thermodynamics Study
2.3.4. Desorption and Regeneration Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Silk Sericin and Kraft Lignin Blend Beads
3.3. Characterization of the SS/KL Beads
3.4. Batch Adsorption Studies
3.5. Desorption and Regeneration Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- De Souza, F.B.; de Lima Brandão, H.; Hackbarth, F.V.; de Souza, A.A.U.; Boaventura, R.A.R.; de Souza Selene, M.A.G.U.; Vilar, V.J.P. Marine macro-alga Sargassum cymosum as electron donor for hexavalent chromium reduction to trivalent state in aqueous solutions. Chem. Eng. J. 2016, 283, 903–910. [Google Scholar] [CrossRef]
- Barry, D.M.; Kanematsu, H. World Health Organization’s standards from the viewpoint of health risks. In Corrosion Control and Surface Finishing; Springer: Tokyo, Japan, 2016; pp. 79–88. [Google Scholar]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. Silk sericin retards the crystallization of silk fibroin. Macromol. Rapid Commun. 2004, 25, 1792–1796. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Statistics. Available online: http://inserco.org/en/?q=statistics (accessed on 29 June 2016).
- Kanazawa, T.; Shizawa, Y.; Takeuchi, M.; Tamano, K.; Ibaraki, H.; Seta, Y.; Takashima, Y.; Okada, H. Topical anti-nuclear factor-κB small interfering RNA with functional peptides containing sericin-based hydrogel for atopic dermatitis. Pharmaceutics 2015, 7, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Faragò, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. TNF-α blocker effect of naringenin-loaded sericin microparticles that are potentially useful in the treatment of psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, M.K.; Kwak, H.W.; Lee, J.Y.; Kim, M.H.; Lee, K.H. The role of glycerol and water in flexible silk sericin film. Int. J. Biol. Macromol. 2016, 82, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, M.K.; Kwak, H.W.; Lee, J.Y.; Kim, M.H.; Kim, E.H.; Lee, K.H. Preparation and characterization of silk sericin/glycerol/graphene oxide nanocomposite film. Fibers Polym. 2013, 14, 2111–2116. [Google Scholar] [CrossRef]
- Nishida, A.; Yamada, M.; Kanazawa, T.; Takashima, Y.; Ouchi, K.; Okada, H. Sustained-release of protein from biodegradable sericin film, gel and sponge. Int. J. Pharm. 2011, 407, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Zhang, J.; Sun, N.; Huang, K.; Li, H.; Wang, Z.; Huang, K.; Wang, L. An injectable silk sericin hydrogel promotes cardiac functional recovery after ischemic myocardial infarction. Acta Biomater. 2016, 41, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kurland, N.E.; Ragland, R.B.; Zhang, A.; Moustafa, M.E.; Kundu, S.C.; Yadavalli, V.K. PH responsive poly amino-acid hydrogels formed via silk sericin templating. Int. J. Biol. Macromol. 2014, 70, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Wantanasiri, P.; Ratanavaraporn, J.; Yamdech, R.; Aramwit, P. Fabrication of silk sericin/alginate microparticles by electrohydrodynamic spraying technique for the controlled release of silk sericin. J. Electrost. 2014, 72, 22–27. [Google Scholar] [CrossRef]
- Zhang, X.; Khan, M.M.R.; Yamamoto, T.; Tsukada, M.; Morikawa, H. Fabrication of silk sericin nanofibers from a silk sericin-hope cocoon with electrospinning method. Int. J. Biol. Macromol. 2012, 50, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tsukada, M.; Morikawa, H.; Aojima, K.; Zhang, G.; Miura, M. Production of silk sericin/silk fibroin blend nanofibers. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ki, C.S.; Um, I.C.; Park, Y.H. Acceleration effect of sericin on shear-induced β-transition of silk fibroin. Polymer 2009, 50, 4618–4625. [Google Scholar] [CrossRef]
- Ki, C.S.; Kim, J.W.; Oh, H.J.; Lee, K.H.; Park, Y.H. The effect of residual silk sericin on the structure and mechanical property of regenerated silk filament. Int. J. Biol. Macromol. 2007, 41, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.N.; Um, I.C. Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int. J. Biol. Macromol. 2015, 78, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.Y.; Kim, A.; Ki, C.S.; Kim, J.W.; Park, Y.H.; Lee, K.H. Preparation of silk sericin beads using LiCl/DMSO solvent and their potential as a drug carrier for oral administration. Fibers Polym. 2007, 8, 470–476. [Google Scholar] [CrossRef]
- Oh, H.; Kim, M.K.; Lee, K.H. Preparation of sericin microparticles by electrohydrodynamic spraying and their application in drug delivery. Macromol. Res. 2011, 19, 266–272. [Google Scholar] [CrossRef]
- Kwak, H.W.; Kim, Y.; Yun, N.K.; Lee, K.H. Silk sericin microparticles as a biosorbent for hexavalent chromium ion. Macromol. Res. 2014, 22, 788–795. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, S.H.; Kim, J.H.; Yu, H.; Kim, H.J.; Yang, Y.-H.; Kim, H.; Kim, Y.H.; Ha, S.H.; Lee, S.H. Application of cellulose/lignin hydrogel beads as novel supports for immobilizing lipase. J. Mol. Catal. B Enzym. 2015, 119, 33–39. [Google Scholar] [CrossRef]
- Salas, C.; Ago, M.; Lucia, L.A.; Rojas, O.J. Synthesis of soy protein–lignin nanofibers by solution electrospinning. React. Funct. Polym. 2014, 85, 221–227. [Google Scholar] [CrossRef]
- Ma, X.; Kolla, P.; Zhao, Y.; Smirnova, A.L.; Fong, H. Electrospun lignin-derived carbon nanofiber mats surface-decorated with MnO2 nanowhiskers as binder-free supercapacitor electrodes with high performance. J. Power Sources 2016, 325, 541–548. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Yan, N.; Zhang, R.; Li, J. Demethylation of wheat straw alkali lignin for application in phenol formaldehyde adhesives. Polymers 2016, 8, 209. [Google Scholar] [CrossRef]
- Wang, J.; Vermerris, W. Antimicrobial nanomaterials derived from natural products—A review. Materials 2016, 9, 255. [Google Scholar] [CrossRef]
- Faruk, O.; Obaid, N.; Tjong, J.; Sain, M. Lignin reinforcement in thermoplastic composites. In Lignin in Polymer Composites; William Andrew Publishing: Toronto, ON, Canada, 2016; pp. 95–118. [Google Scholar]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose–lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Klapiszewski, L.; Bartczak, P.; Wysokowski, M.; Jankowska, M.; Kabat, K.; Jesionowski, T. Silica conjugated with kraft lignin and its use as a novel ‘green’ sorbent for hazardous metal ions removal. Chem. Eng. J. 2015, 260, 684–693. [Google Scholar] [CrossRef]
- Wysokowski, M.; Klapiszewski, L.; Moszynski, D.; Bartczak, P.; Szatkowsk, T.; Majchrzak, I.; Siwińska-Stefańska, K.; Bazhenov, V.; Jesionowski, T. Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium(II) and nickel(II) ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed]
- Harmita, H.; Karthikeyan, K.G.; Pan, X. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan (chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Kwak, H.W.; Kim, M.K.; Lee, J.Y.; Yun, H.; Kim, M.H.; Park, Y.H.; Lee, K.H. Preparation of bead-type biosorbent from water-soluble Spirulina platensis extracts for chromium (VI) removal. Algal Res. 2015, 7, 92–99. [Google Scholar] [CrossRef]
- Popuri, S.R.; Vijaya, Y.; Boddu, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Sreekala, M.S.; Thomas, S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485. [Google Scholar] [CrossRef]
- Saraf, V.P.; Glasser, W.G. Engineering plastics from lignin. III. Structure property relationships in solution cast polyurethane films. J. Appl. Polym. Sci. 1984, 29, 1831–1841. [Google Scholar] [CrossRef]
- Wang, K.; Loo, L.S.; Goh, K.L. A facile method for processing lignin reinforced chitosan biopolymer microfibres: Optimising the fibre mechanical properties through lignin type and concentration. Mater. Res. Express 2016, 3, 035301. [Google Scholar] [CrossRef]
- Bolte, M.; Israeli, Y.; Djouani, F.; Rivaton, A.; Frezet, L.; Lessard, R.A. Hologram formation reconsidered in dichromated polyvinylalcohol: Polymer cross-linking around chromium(V). Proc. SPIE 2005, 5742, 195–204. [Google Scholar]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Bailey, J.E.; Ollis, D.F. Biochemical engineering fundamentals. Chem. Eng. Educ. 1976, 10, 162–165. [Google Scholar]
- Imai, A.; Gloyna, E.F. Effects of pH and oxidation state of chromium on the behavior of chromium in the activated sludge process. Water Res. 1990, 24, 1143–1150. [Google Scholar] [CrossRef]
- Dai, J.; Yan, H.; Yang, H.; Cheng, R. Simple method for preparation of chitosan/poly(acrylic acid) blending hydrogel beads and adsorption of copper(II) from aqueous solutions. Chem. Eng. J. 2010, 165, 240–249. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, W.; Plazinski, W. Kinetics of solute adsorption at solid/solution interfaces: A theoretical development of the empirical pseudo-first and pseudo-second order kinetic rate equations, based on applying the statistical rate theory of interfacial transport. J. Phys. Chem. B 2006, 110, 16514–16525. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Xu, Y.; Lu, S.; Ju, S.; Xing, W. Adsorption of cefocelis hydrochloride on macroporous resin: Kinetics, Equilibrium, and thermodynamic studies. J. Chem. Eng. Data 2016, 61, 2179–2185. [Google Scholar] [CrossRef]













| Blend Ratio (Sericin/Lignin) | Elemental Analysis Data Calculated Value (%) | |||
|---|---|---|---|---|
| C | H | N | S | |
| 100:0 | 39.39 | 6.42 | 14.64 | 0.40 |
| 90:10 | 40.84 | 6.28 | 12.53 | 0.52 |
| 80:20 | 41.35 | 6.42 | 12.06 | 0.64 |
| 70:30 | 42.51 | 6.53 | 11.33 | 0.80 |
| 60:40 | 44.40 | 6.52 | 10.67 | 0.92 |
| 50:50 | 46.78 | 6.50 | 8.96 | 1.04 |
| 40:60 | 45.21 | 6.30 | 9.76 | 1.01 |
| 30:70 | 45.33 | 6.44 | 9.56 | 0.94 |
| Kraft lignin powder | 61.60 | 6.27 | 0.50 | 1.75 |
| Parameter | Value | R2 |
|---|---|---|
| Langmuir isotherm | – | 0.916 |
| Q (mg/g) | 139.86 | |
| KL (L/mg) | 0.074 | |
| Freundlich isotherm | – | 0.999 |
| n (L/mg) | 2.05 | |
| Kf (mg/g) | 14.72 | |
| BET isotherm | – | 0.952 |
| Q (mg/g) | 250.10 | |
| B (g/mg) | 2.26 × 105 |
| C0 (mg/L) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| 100 | K1 (min−1) | qe (mg·g−1) | R2 | K2 × 10−3 (g·mg−1·min−1) | qe (mg·g−1) | R2 |
| 2.65 × 103 | 53.62 | 0.791 | 0.0137 | 53.16 | 0.998 | |
| Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol·K) |
|---|---|---|---|
| 293 | −0.511 | 10.53 | 0.121 |
| 303 | −2.133 | ||
| 313 | −2.827 | ||
| 323 | −4.300 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, H.W.; Shin, M.; Yun, H.; Lee, K.H. Preparation of Silk Sericin/Lignin Blend Beads for the Removal of Hexavalent Chromium Ions. Int. J. Mol. Sci. 2016, 17, 1466. https://doi.org/10.3390/ijms17091466
Kwak HW, Shin M, Yun H, Lee KH. Preparation of Silk Sericin/Lignin Blend Beads for the Removal of Hexavalent Chromium Ions. International Journal of Molecular Sciences. 2016; 17(9):1466. https://doi.org/10.3390/ijms17091466
Chicago/Turabian StyleKwak, Hyo Won, Munju Shin, Haesung Yun, and Ki Hoon Lee. 2016. "Preparation of Silk Sericin/Lignin Blend Beads for the Removal of Hexavalent Chromium Ions" International Journal of Molecular Sciences 17, no. 9: 1466. https://doi.org/10.3390/ijms17091466