Dispersion of Nanoparticles in Lubricating Oil: A Critical Review
Abstract
:1. Introduction
2. Dispersion Methods
2.1. Formulation with Dispersant
2.2. Surface Modification
2.2.1. Surfactant
2.2.2. Silanization
2.3. Other Methods
2.4. Evaluation
3. Theory
3.1. Some Basics
3.2. Steric Stabilization
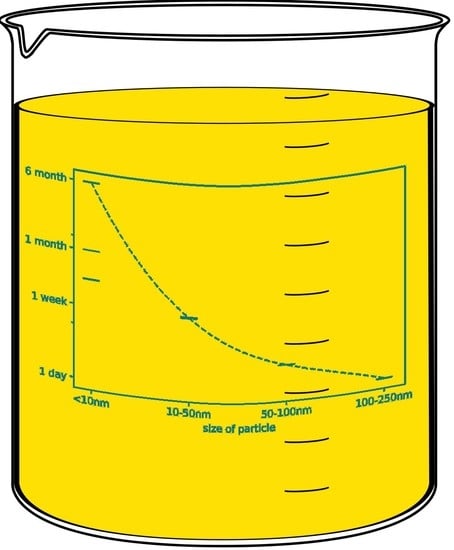
3.3. Size Effects
3.4. Grafted Surface Layers
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef] [Green Version]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Nylund, N.-O.; Mäkelä, K.; Erdemir, A. Global energy consumption due to friction in trucks and buses. Tribol. Int. 2014, 78, 94–114. [Google Scholar] [CrossRef]
- Holmberg, K.; Kivikytö-Reponen, P.; Härkisaari, P.; Valtonen, K.; Erdemir, A. Global energy consumption due to friction and wear in the mining industry. Tribol. Int. 2017, 115, 116–139. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Zulkifli, N.W.M.; Mufti, R.A. Tribological performance of nanoparticles as lubricating oil additives. J. Nanoparticle Res. 2016, 18, 223. [Google Scholar] [CrossRef]
- Chinas-Castillo, F.; Spikes, H.A. Mechanism of action of colloidal solid dispersions. J. Tribol. 2003, 125, 552–557. [Google Scholar] [CrossRef]
- Kalin, M.; Kogovšek, J.; Remškar, M. Mechanisms and improvements in the friction and wear behavior using MoS2 nanotubes as potential oil additives. Wear 2012, 280–281, 36–45. [Google Scholar] [CrossRef]
- Rabaso, P.; Ville, F.; Dassenoy, F.; Diaby, M.; Afanasiev, P.; Cavoret, J.; Vacher, B.; Le Mogne, T. Boundary lubrication: Influence of the size and structure of inorganic fullerene-like MoS2 nanoparticles on friction and wear reduction. Wear 2014, 320, 161–178. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Liu, W.; Xue, Q. Tribochemistry and antiwear mechanism of organic–inorganic nanoparticles as lubricant additives. Tribol. Lett. 2006, 22, 79–84. [Google Scholar] [CrossRef]
- Wright, R.A.E.; Wang, K.; Qu, J.; Zhao, B. Oil-Soluble Polymer Brush Grafted Nanoparticles as Effective Lubricant Additives for Friction and Wear Reduction. Angew. Chem. Int. Ed. 2016, 55, 8656–8660. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, L.; Zuenkeler, S.; Toensing, K.; Anselmetti, D. An Oil-Based Lubrication System Based on Nanoparticular TiO2 with Superior Friction and Wear Properties. Tribol. Lett. 2015, 59, 29. [Google Scholar] [CrossRef]
- Kim, D.; Archer, L.A. Nanoscale Organic−Inorganic Hybrid Lubricants. Langmuir 2011, 27, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.T.; Wright, R.A.; Parrott, A.C.; Gao, H.; Martini, A.; Qu, J.; Dei, S.; Zhao, B. Poly (alkyl methacrylate) Brush-Grafted Silica Nanoparticles as Oil Lubricant Additives: Effects of Alkyl Pendant Groups on Oil Dispersibility, Stability, and Lubrication Property. ACS Appl. Mater. Interfaces 2017, 9, 25038–25048. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jiang, B.; He, J.; Xia, X.; Pan, F. Lubrication performance of MoS2 and SiO2 nanoparticles as lubricant additives in magnesium alloy-steel contacts. Tribol. Int. 2016, 93, 63–70. [Google Scholar] [CrossRef]
- Sui, T.; Song, B.; Zhang, F.; Yang, Q. Effect of Particle Size and Ligand on the Tribological Properties of Amino Functionalized Hairy Silica Nanoparticles as an Additive to Polyalphaolefin. J. Nanomater. 2015, 16, 427. [Google Scholar] [CrossRef]
- López, T.D.-F.; González, A.F.; Reguero, Á.D.; Matos, M.; Díaz-García, M.E.; Badía-Laíño, R. Engineered silica nanoparticles as additives in lubricant oils. Sci. Technol. Adv. Mater. 2015, 16, 055005. [Google Scholar] [CrossRef] [Green Version]
- Sui, T.; Song, B.; Zhang, F.; Yang, Q. Effects of functional groups on the tribological properties of hairy silica nanoparticles as an additive to polyalphaolefin. RSC Adv. 2016, 6, 393–402. [Google Scholar] [CrossRef]
- Jiao, D.; Zheng, S.; Wang, Y.; Guan, R.; Cao, B. The tribology properties of alumina/silica composite nanoparticles as lubricant additives. Appl. Surf. Sci. 2011, 257, 5720–5725. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, S.; Yang, S.; Li, W.; Song, X.; Cao, B. Enhanced tribology properties of ZnO/Al2O3 composite nanoparticles as liquid lubricating additives. J. Sol-Gel Sci. Technol. 2012, 61, 501–508. [Google Scholar] [CrossRef]
- Luo, T.; Wei, X.; Huang, X.; Huang, L.; Yang, F. Tribological properties of Al2O3 nanoparticles as lubricating oil additives. Ceram. Int. 2014, 40, 7143–7149. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, J.; Wang, J.; Yang, Z.; Liu, S.; Wang, Z.; Yang, S. Preparation of a reduced graphene oxide/zirconia nanocomposite and its application as a novel lubricant oil additive. RSC Adv. 2015, 5, 91802–91812. [Google Scholar] [CrossRef]
- Shahar, C.; Zbaida, D.; Rapoport, L.; Cohen, H.; Bendikov, T.; Tannous, J. Surface Functionalization of WS2 Fullerene-like Nanoparticles. Langmuir 2010, 26, 4409–4414. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Y.; Yang, G.; Yang, K.; Zhang, S.; Yu, L. Tribological Properties of Oleylamine-Modified Ultrathin WS2 Nanosheets as the Additive in Polyalpha Olefin Over a Wide Temperature Range. Tribol. Lett. 2016, 61, 24. [Google Scholar] [CrossRef]
- Wu, J.; Fu, X. A low-temperature solvothermal method to prepare hollow spherical WS2 nanoparticles modified by TOA. Mater. Lett. 2007, 61, 4332–4335. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Xu, Y.; Hu, K.H.; Xu, Y.F.; Hu, X.G. Formation and tribological properties of hollow sphere-like nano-MoS2 precipitated in TiO2 particles. Tribol. Int. 2015, 81, 139–148. [Google Scholar] [CrossRef]
- Zin, V.; Agresti, F.; Barison, S.; Colla, L.; Mercadelli, E.; Fabrizio, M.; Pagura, M. Tribological Properties of Engine Oil with Carbon Nano-horns as Nano-additives. Tribol. Lett. 2014, 55, 45–53. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Courier Corporation: Chelmsford, MA, USA, 2000. [Google Scholar]
- Lin, J.; Wang, L.; Chen, G. Modification of Graphene Platelets and their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Einstein, A. Investigations on the Theory of the Brownian Movement; Courier Corporation: Chelmsford, MA, USA, 1956. [Google Scholar]
- van Roij, R. Defying gravity with entropy and electrostatics: Sedimentation of charged colloids. J. Phys. Condens. Matter. 2003, 15, S3569. [Google Scholar] [CrossRef]
- Piazza, R. Settled and unsettled issues in particle settling. Rep. Prog. Phys. 2014, 77, 056602. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, W.; Zhang, Z. Friction and wear properties of a surface-modified TiO2 nanoparticle as an additive in liquid paraffin. Wear 1997, 213, 29–32. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Wan, H.; Chen, J.; Zhou, H. Synthesis and Tribological Properties of Stearic Acid-Modified Anatase (TiO2) Nanoparticles. Tribol. Lett. 2011, 41, 409–416. [Google Scholar] [CrossRef]
- Ingole, S.; Charanpahari, A.; Kakade, A.; Umare, S.S.; Bhatt, D.V.; Menghani, J. Tribological behavior of nano TiO2 as an additive in base oil. Wear 2013, 301, 776–785. [Google Scholar] [CrossRef]
- Beel, A.; Gottschalk, M.; Huetten, A.; Toensing, K.; Anselmetti, D. Tribological Performance of TiO2-Nanostructured Particles as Oil-Lubricant Additives for Different Iron-Carbon Alloys. Mater. Today Proc. 2017, 4, S75–S80. [Google Scholar] [CrossRef]
- Kheireddin, B.A.; Lu, W.; Chen, I.-C.; Akbulut, M. Inorganic nanoparticle-based ionic liquid lubricants. Wear 2013, 303, 185–190. [Google Scholar] [CrossRef]
- Seymour, B.T.; Fu, W.; Wright, R.A.E.; Luo, H.; Qu, J.; Dai, S.; Zhao, B. Improved Lubricating Performance by Combining Oil-Soluble Hairy Silica Nanoparticles and an Ionic Liquid as an Additive for a Synthetic Base Oil. ACS Appl. Mater. Interfaces 2018, 10, 15129–15139. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, Z.; Zhang, Z.; Dang, H. Surface-modification in situ of nano-SiO2 and its structure and tribological properties. Appl. Surf. Sci. 2006, 252, 7856–7861. [Google Scholar] [CrossRef]
- Sui, T.; Ding, M.; Ji, C.; Yan, S.; Wei, J.; Wang, A.; Zhao, F. Dispersibility and rheological behavior of functionalized silica nanoparticles as lubricant additives. Ceram Int. 2018. [Google Scholar] [CrossRef]
- Sui, T.; Song, B.; Wen, Y.; Zhang, F. Bifunctional hairy silica nanoparticles as high-performance additives for lubricant. Sci. Rep. 2016, 6, 22696. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zheng, S.; Cao, B.; Ma, S. Friction and wear properties of ZrO2/SiO2 composite nanoparticles. J. Nanoparticle Res. 2011, 13, 2129–2137. [Google Scholar] [CrossRef]
- Alves, S.M.; Mello, V.S.; Faria, E.A.; Camargo, A.P.P. Nanolubricants developed from tiny CuO nanoparticles. Tribol. Int. 2016, 100, 263–271. [Google Scholar] [CrossRef]
- Hernández Battez, A.; González, R.; Viesca, J.L.; Fernández, J.E.; Díaz Fernández, J.M.; Machado, A.; Chou, R.; Riba, J. CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 2008, 265, 422–428. [Google Scholar] [CrossRef]
- Alves, S.M.; Barros, B.S.; Trajano, M.F.; Ribeiro, K.S.B.; Moura, E. Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribol. Int. 2013, 65, 28–36. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, Y.; Wang, X.; Xia, M.; Zhang, Y.; Ding, H. Sliding tribological properties of 0.45% carbon steel lubricated with Fe3O4 magnetic nano-particle additives in baseoil. Wear 2013, 301, 753–757. [Google Scholar] [CrossRef]
- Hu, Z.S.; Dong, J.X.; Chen, G.X. Study on antiwear and reducing friction additive of nanometer ferric oxide. Tribol. Int. 1998, 31, 355–360. [Google Scholar] [CrossRef]
- Luo, T.; Wei, X.; Zhao, H.; Cai, G.; Zheng, X. Tribology properties of Al2O3/TiO2 nanocomposites as lubricant additives. Ceram Int. 2014, 40, 10103–10109. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W.; Yu, L. Preparation of DDP-coated PbS nanoparticles and investigation of the antiwear ability of the prepared nanoparticles as additive in liquid paraffin. Wear 1998, 218, 153–158. [Google Scholar] [CrossRef]
- Rosentsveig, R.; Gorodnev, A.; Feuerstein, N.; Friedman, H.; Zak, A.; Fleischer, N.; Tannous, J.; Dassenoy, F.; Tenne, R. Fullerene-like MoS2 Nanoparticles and Their Tribological Behavior. Tribol. Lett. 2009, 36, 175–182. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Dassenoy, F.; Belin, M.; Vacher, B.; Martin, J.M.; Fleischer, N. Ultralow-friction and wear properties of IF-WS2 under boundary lubrication. Tribol. Lett. 2005, 18, 477–485. [Google Scholar] [CrossRef]
- Moshkovith, A.; Perfiliev, V.; Verdyan, A.; Lapsker, I.; Popovitz-Biro, R.; Tenne, R. Sedimentation of IF-WS2 aggregates and a reproducibility of the tribological data. Tribol. Int. 2007, 40, 117–124. [Google Scholar] [CrossRef]
- Jifen, W.; Wensheng, Z.; Guifen, J. Preparation and tribological properties of tungsten disulfide hollow spheres assisted by methyltrioctylammonium chloride. Tribol. Int. 2010, 43, 1650–1658. [Google Scholar] [CrossRef]
- Cizaire, L.; Vacher, B.; Le Mogne, T.; Martin, J.M.; Rapoport, L.; Margolin, A.; Tenne, R. Mechanisms of ultra-low friction by hollow inorganic fullerene-like MoS2 nanoparticles. Surf. Coat. Technol. 2002, 160, 282–287. [Google Scholar] [CrossRef]
- Wu, X.; Gong, K.; Zhao, G.; Lou, W.; Wang, X.; Liu, W. MoS2/WS2 Quantum Dots as High-Performance Lubricant Additive in Polyalkylene Glycol for Steel/Steel Contact at Elevated Temperature. Adv. Mater. Interfaces 2018, 5, 1700859. [Google Scholar] [CrossRef]
- Bakunin, V.N.; Kuzmina, G.N.; Kasrai, M.; Parenago, O.P.; Bancroft, G.M. Tribological behavior and tribofilm composition in lubricated systems containing surface-capped molybdenum sulfide nanoparticles. Tribol. Lett. 2006, 22, 289–296. [Google Scholar] [CrossRef]
- Koshy, C.P.; Rajendrakumar, P.K.; Thottackkad, M.V. Evaluation of the tribological and thermo-physical properties of coconut oil added with MoS2 nanoparticles at elevated temperatures. Wear 2015, 330–331, 288–308. [Google Scholar] [CrossRef]
- Wu, X.; Gong, K.; Zhao, G.; Lou, W.; Wang, X.; Liu, W. Surface Modification of MoS2 Nanosheets as Effective Lubricant Additives for Reducing Friction and Wear in Poly-α-olefin. Ind. Eng. Chem. Res. 2018, 57, 8105–8114. [Google Scholar] [CrossRef]
- Fernández-Coppel, I.A.; Martín-Ramos, P.; Martín-Gil, J.; Pamies, R.; Avella, M.; Avilés, M.D. Synthesis and Exploration of the Lubricating Behavior of Nanoparticulated Mo15S19 in Linseed Oil. Materials 2018, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, B.; Zhu, L.; Zhu, H. Synthesis and tribological property study of oleic acid-modified copper sulfide nanoparticles. Wear 2008, 265, 150–154. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Zhang, Z.; Liu, W.; Xue, Q. Tribological behavior and lubricating mechanism of Cu nanoparticles in oil. Tribol. Lett. 2000, 8, 213–218. [Google Scholar] [CrossRef]
- Yang, G.; Chai, S.; Xiong, X.; Zhang, S.; Yu, L.; Zhang, P. Preparation and tribological properties of surface modified Cu nanoparticles. Trans. Nonferrous Met. Soc. China 2012, 22, 366–372. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, C.; Hwang, Y.; Park, M.; Lee, J.; Choi, C.; Jung, M. Tribological behavior of copper nanoparticles as additives in oil. Curr. Appl. Phys. 2009, 9, e124–e127. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Qin, B.; Xing, D.; Guo, Y.; Fan, R. Investigation of the Mending Effect and Mechanism of Copper Nano-Particles on a Tribologically Stressed Surface. Tribol. Lett. 2004, 17, 961–966. [Google Scholar] [CrossRef]
- Viesca, J.L.; Hernández Battez, A.; González, R.; Chou, R.; Cabello, J.J. Antiwear properties of carbon-coated copper nanoparticles used as an additive to a polyalphaolefin. Tribol. Int. 2011, 44, 829–833. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Ding, Q.; Li, H.; Qin, B.; Hu, L. Understanding the synergistic lubrication effect of 2-mercaptobenzothiazolate based ionic liquids and Mo nanoparticles as hybrid additives. Tribol. Int. 2018, 125, 39–45. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, S.; Yu, L.; Zhang, P.; Zhang, Z. Preparation of Nickel-Based Nanolubricants via a Facile In Situ One-Step Route and Investigation of Their Tribological Properties. Tribol. Lett. 2013, 51, 73–83. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Z.; Dong, J.; Chen, G. Preparation of Ni nanoparticles and evaluation of their tribological performance as potential additives in oils. J. Tribol. 2001, 123, 441–443. [Google Scholar] [CrossRef]
- Chou, R.; Battez, A.H.; Cabello, J.J.; Viesca, J.L.; Osorio, A.; Sagastume, A. Tribological behavior of polyalphaolefin with the addition of nickel nanoparticles. Tribol. Int. 2010, 43, 2327–2332. [Google Scholar] [CrossRef]
- Kumara, C.; Luo, H.; Leonard, D.N.; Meyer, H.M.; Qu, J. Organic-Modified Silver Nanoparticles as Lubricant Additives. ACS Appl. Mater. Interfaces 2017, 9, 37227–37237. [Google Scholar] [CrossRef] [PubMed]
- Ghaednia, H.; Hossain, M.S.; Jackson, R.L. Tribological Performance of Silver Nanoparticle–Enhanced Polyethylene Glycol Lubricants. Tribol. Trans. 2016, 59, 585–592. [Google Scholar] [CrossRef]
- Li, D.; Hong, B.; Fang, W.; Guo, Y.; Lin, R. Preparation of Well-Dispersed Silver Nanoparticles for Oil-Based Nanofluids. Ind. Eng. Chem. Res. 2010, 49, 1697–1702. [Google Scholar] [CrossRef]
- Sun, L.; Tao, X.; Zhao, Y.; Zhang, Z. Synthesis and Tribology Properties of Stearate-Coated Ag Nanoparticles. Tribol. Trans. 2010, 53, 174–178. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.J.; Wu, Z.S.; Dang, H.X. Synthesis and characterization of DDP coated Ag nanoparticles. Mater. Sci. Eng. A 2004, 379, 378–383. [Google Scholar] [CrossRef]
- Kolodziejczyk, L.; Martínez-Martínez, D.; Rojas, T.C.; Fernández, A.; Sánchez-López, J.C. Surface-modified Pd nanoparticles as a superior additive for lubrication. J. Nanoparticle Res. 2007, 9, 639–645. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Abad, M.D.; Kolodziejczyk, L.; Guerrero, E.; Fernández, A. Surface-modified Pd and Au nanoparticles for anti-wear applications. Tribol. Int. 2011, 44, 720–726. [Google Scholar] [CrossRef]
- Kumara, C.; Leonard, D.N.; Meyer, H.M.; Luo, H.; Armstrong, B.L.; Qu, J. Palladium Nanoparticle-Enabled Ultrathick Tribofilm with Unique Composition. ACS Appl. Mater. Interfaces 2018, 10, 31804–31812. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Dang, H. Fabrication and Tribological Properties of Pb Nanoparticles. J. Nanoparticle Res. 2004, 6, 47–51. [Google Scholar] [CrossRef]
- Huang, H.D.; Tu, J.P.; Gan, L.P.; Li, C.Z. An investigation on tribological properties of graphite nanosheets as oil additive. Wear 2006, 261, 140–144. [Google Scholar] [CrossRef]
- Sanes, J.; Avilés, M.-D.; Saurín, N.; Espinosa, T.; Carrión, F.-J.; Bermúdez, M.-D. Synergy between graphene and ionic liquid lubricant additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. Appl. Phys. 2011, 44, 205303. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 2012, 22, 21032–21039. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.; Song, H.; Chen, B.; Zhang, Z. Synthesis of the liquid-like graphene with excellent tribological properties. Tribol. Int. 2017, 105, 118–124. [Google Scholar] [CrossRef]
- Sarno, M.; Senatore, A.; Cirillo, C.; Petrone, V.; Ciambelli, P. Oil Lubricant Tribological Behaviour Improvement Through Dispersion of Few Layer Graphene Oxide. J. Nanosci. Nanotechnol. 2014. [Google Scholar] [CrossRef]
- Kumar, P.; Wani, M.F. Tribological Characterisation of Graphene Oxide as Lubricant Additive on Hypereutectic Al-25Si/Steel Tribopair. Tribol. Trans. 2018, 61, 335–346. [Google Scholar] [CrossRef]
- Kinoshita, H.; Ono, H.; Alias, A.A.; Nishina, Y.; Fujii, M. Tribological properties of graphene oxide as a lubricating additive in water and lubricating oils. Mech. Eng. J. 2015, 2. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Etacheri, V.; Dysart, A.D.; Stacke, L.-E.; Pol, V.G.; Sadeghi, F. Ultrasmooth Submicrometer Carbon Spheres as Lubricant Additives for Friction and Wear Reduction. ACS Appl. Mater. Interfaces 2015, 7, 5514–5521. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Jiazheng, Z.; Kang, X. The ball-bearing effect of diamond nanoparticles as an oil additive. J. Phys. Appl. Phys. 1996, 29, 2932. [Google Scholar] [CrossRef]
- Marko, M.; Kyle, J.; Branson, B.; Terrell, E. Tribological Improvements of Dispersed Nanodiamond Additives in Lubricating Mineral Oil. J. Tribol. 2014, 137, 011802. [Google Scholar] [CrossRef]
- Acharya, B.; Avva, K.S.; Thapa, B.; Pardue, T.N.; Krim, J. Synergistic Effect of Nanodiamond and Phosphate Ester Anti-Wear Additive Blends. Lubricants 2018, 6, 56. [Google Scholar] [CrossRef]
- Chou, C.-C.; Lee, S.-H. Tribological behavior of nanodiamond-dispersed lubricants on carbon steels and aluminum alloy. Wear 2010, 269, 757–762. [Google Scholar] [CrossRef]
- Lee, J.; Cho, S.; Hwang, Y.; Lee, C.; Kim, S.H. Enhancement of Lubrication Properties of Nano-oil by Controlling the Amount of Fullerene Nanoparticle Additives. Tribol. Lett. 2007, 28, 203–208. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Vacher, B.; Ohmae, N.; Martin, J.M.; Epicier, T. Anti-wear and Friction Reducing Mechanisms of Carbon Nano-onions as Lubricant Additives. Tribol. Lett. 2008, 30, 69–80. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Bucholz, E.W.; Matsumoto, N.; Phillpot, S.R.; Sinnott, S.B.; Ohmae, N. Friction Properties of Carbon Nano-Onions from Experiment and Computer Simulations. Tribol. Lett. 2009, 37, 75. [Google Scholar] [CrossRef]
- Matsumoto, N.; Mistry, K.K.; Kim, J.-H.; Eryilmaz, O.L.; Erdemir, A.; Kinoshita, H. Friction reducing properties of onion-like carbon based lubricant under high contact pressure. Tribol. Mater. Surf. Interfaces 2012, 6, 116–120. [Google Scholar] [CrossRef]
- Nunn, N.; Mahbooba, Z.; Ivanov, M.G.; Ivanov, D.M.; Brenner, D.W.; Shenderova, O. Tribological properties of polyalphaolefin oil modified with nanocarbon additives. Diam. Relat. Mater. 2015, 54, 97–102. [Google Scholar] [CrossRef]
- Cornelio, J.A.C.; Cuervo, P.A.; Hoyos-Palacio, L.M.; Lara-Romero, J.; Toro, A. Tribological properties of carbon nanotubes as lubricant additive in oil and water for a wheel–rail system. J. Mater. Res. Technol. 2016, 5, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Khalil, W.; Mohamed, A.; Bayoumi, M.; Osman, T.A. Tribological properties of dispersed carbon nanotubes in lubricant. Fuller Nanotub. Carbon Nanostruct. 2016, 24, 479–485. [Google Scholar] [CrossRef]
- Salah, N.; Abdel-wahab, M.S.; Habib, S.S.; Khan, Z.H. Lubricant Additives Based on Carbon Nanotubes Produced from Carbon-Rich Fly Ash. Tribol. Trans. 2017, 60, 166–175. [Google Scholar] [CrossRef]
- Pamies, R.; Espejo, C.; Carrión, F.J.; Morina, A.; Neville, A.; Bermúdez, M.D. Rheological behavior of multiwalled carbon nanotube-imidazolium tosylate ionic liquid dispersions. J. Rheol. 2017, 61, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ren, R.; Song, H.; Jia, X. Improved tribological properties of the synthesized copper/carbon nanotube nanocomposites for rapeseed oil-based additives. Appl. Surf. Sci. 2018, 428, 630–639. [Google Scholar] [CrossRef]
- Qiu, S.; Dong, J.; Chen, G. Wear and friction behaviour of CaCO3 nanoparticles used as additives in lubricating oils. Lubr. Sci. 2000, 12, 205–212. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Fu, X.; Xia, Y. Performance and anti-wear mechanism of CaCO3 nanoparticles as a green additive in poly-alpha-olefin. Tribol. Int. 2009, 42, 1029–1039. [Google Scholar] [CrossRef]
- Peri, J.B. The state of dispersion of detergent additives in lubricating oil and other hydrocarbons. J. Am. Oil Chem. Soc. 1958, 35, 110–117. [Google Scholar] [CrossRef]
- Dong, J.X.; Hu, Z.S. A study of the anti-wear and friction-reducing properties of the lubricant additive, nanometer zinc borate. Tribol. Int. 1998, 31, 219–223. [Google Scholar] [CrossRef]
- Song, X.; Zheng, S.; Zhang, J.; Li, W.; Chen, Q.; Cao, B. Synthesis of monodispersed ZnAl2O4 nanoparticles and their tribology properties as lubricant additives. Mater. Res. Bull. 2012, 47, 4305–4310. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Xue, Q. Study on lubricating mechanisms of La(OH)3 nanocluster modified by compound containing nitrogen in liquid paraffin. Wear 1998, 218, 139–144. [Google Scholar] [CrossRef]
- Hu, Z.S.; Dong, J.X.; Chen, G.X.; He, J.Z. Preparation and tribological properties of nanoparticle lanthanum borate. Wear 2000, 243, 43–47. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Zhang, Z.; Liu, W.; Dang, H. Study on an antiwear and extreme pressure additive of surface coated LaF3 nanoparticles in liquid paraffin. Wear 2001, 249, 333–337. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, O.P.; Gusain, R.; Mungse, H.P.; Kukrety, A.; Kumar, N.; Sugimura, H. Alkyl-Chain-Grafted Hexagonal Boron Nitride Nanoplatelets as Oil-Dispersible Additives for Friction and Wear Reduction. ACS Appl. Mater. Interfaces 2015, 7, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Farrokhpay, S. A review of polymeric dispersant stabilisation of titania pigment. Adv. Colloid Interface Sci. 2009, 151, 24–32. [Google Scholar] [CrossRef]
- Rabaso, P.; Dassenoy, F.; Ville, F.; Diaby, M.; Vacher, B.; Mogne, T.L.; Belin, M. An Investigation on the Reduced Ability of IF-MoS2 Nanoparticles to Reduce Friction and Wear in the Presence of Dispersants. Tribol. Lett. 2014, 55, 503–516. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation of TiO2 nanoparticles surface-modified by both carboxylic acid and amine: Dispersibility and stabilization in organic solvents. Colloids Surf. Physicochem. Eng. Asp. 2008, 317, 543–550. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y. Surface-modification of SiO2 nanoparticles with oleic acid. Appl. Surf. Sci. 2003, 211, 315–320. [Google Scholar] [CrossRef]
- Li, C.-C.; Chang, M.-H. Colloidal stability of CuO nanoparticles in alkanes via oleate modifications. Mater. Lett. 2004, 58, 3903–3907. [Google Scholar] [CrossRef]
- Ma, X.; Lee, N.-H.; Oh, H.-J.; Kim, J.-W.; Rhee, C.-K.; Park, K.-S.; Kim, S.-J. Surface modification and characterization of highly dispersed silica nanoparticles by a cationic surfactant. Colloids Surf. Physicochem. Eng. Asp. 2010, 358, 172–176. [Google Scholar] [CrossRef]
- Chang, S.-J.; Liao, W.-S.; Ciou, C.-J.; Lee, J.-T.; Li, C.-C. An efficient approach to derive hydroxyl groups on the surface of barium titanate nanoparticles to improve its chemical modification ability. J. Colloid Interface Sci. 2009, 329, 300–305. [Google Scholar] [CrossRef]
- Du, Y.; Yin, Z.; Zhu, J.; Huang, X.; Wu, X.-J.; Zeng, Z.; Yan, Q.; Zhang, H. A general method for the large-scale synthesis of uniform ultrathin metal sulphide nanocrystals. Nat. Commun. 2012, 3, 1177. [Google Scholar] [CrossRef] [Green Version]
- Bruce, I.J.; Sen, T. Surface Modification of Magnetic Nanoparticles with Alkoxysilanes and Their Application in Magnetic Bioseparations. Langmuir 2005, 21, 7029–7035. [Google Scholar] [CrossRef]
- Vandenberg, E.T.; Bertilsson, L.; Liedberg, B.; Uvdal, K.; Erlandsson, R.; Elwing, H.; Lundström, I. Structure of 3-aminopropyl triethoxy silane on silicon oxide. J. Colloid Interface Sci. 1991, 147, 103–118. [Google Scholar] [CrossRef]
- White, L.D.; Tripp, C.P. Reaction of (3-Aminopropyl)dimethylethoxysilane with Amine Catalysts on Silica Surfaces. J. Colloid Interface Sci. 2000, 232, 400–407. [Google Scholar] [CrossRef]
- Ge, J.; He, L.; Goebl, J.; Yin, Y. Assembly of Magnetically Tunable Photonic Crystals in Nonpolar Solvents. J. Am. Chem. Soc. 2009, 131, 3484–3486. [Google Scholar] [CrossRef]
- Yu, B.; Qian, L.; Yu, J.; Zhou, Z. Effects of Tail Group and Chain Length on the Tribological Behaviors of Self-Assembled Dual-Layer Films in Atmosphere and in Vacuum. Tribol. Lett. 2009, 34, 1. [Google Scholar] [CrossRef]
- Iijima, M.; Tsukada, M.; Kamiya, H. Effect of particle size on surface modification of silica nanoparticles by using silane coupling agents and their dispersion stability in methylethylketone. J. Colloid Interface Sci. 2007, 307, 418–424. [Google Scholar] [CrossRef]
- Pham, K.N.; Fullston, D.; Sagoe-Crentsil, K. Surface Charge Modification of Nano-Sized Silica Colloid. Aust. J. Chem. 2007, 60, 662–666. [Google Scholar] [CrossRef]
- Zeltner, M.; Grass, R.N.; Schaetz, A.; Bubenhofer, S.B.; Luechinger, N.A.; Stark, W.J. Stable dispersions of ferromagnetic carbon-coated metal nanoparticles: Preparation via surface initiated atom transfer radical polymerization. J. Mater. Chem. 2012, 22, 12064–12071. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, L. Mixed Polymer Brush-Grafted Particles: A New Class of Environmentally Responsive Nanostructured Materials. Macromolecules 2009, 42, 9369–9383. [Google Scholar] [CrossRef]
- Rodriguez, R.; Herrera, R.; Archer, L.A.; Giannelis, E.P. Nanoscale Ionic Materials. Adv. Mater. 2008, 20, 4353–4358. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry, 4th ed.; Butterworth-Heinemann: Oxford, UK; Boston, MA, USA, 1992. [Google Scholar]
- Russel, W.B.; Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Van Der Hoeven, P.C.; Lyklema, J. Electrostatic stabilization in non-aqueous media. Adv. Colloid Interface Sci. 1992, 42, 205–277. [Google Scholar] [CrossRef]
- Rubio-Hernández, F.J. Is DLVO Theory Valid for Non-Aqueous Suspensions? J. Non-Equilib. Thermodyn. 2005, 24, 75–79. [Google Scholar] [CrossRef]
- Briscoe, W.H.; Horn, R.G. Direct Measurement of Surface Forces Due to Charging of Solids Immersed in a Nonpolar Liquid. Langmuir 2002, 18, 3945–3956. [Google Scholar] [CrossRef]
- Carey, A.A. The Dielectric Constant of Lubrication Oils, n.d.:9. Available online: www.dtic.mil/docs/citations/ADA347479 (accessed on 10 August 2018).
- Napper, D.H. Steric stabilization. J. Colloid Interface Sci. 1977, 58, 390–407. [Google Scholar] [CrossRef]
- Smitham, J.B.; Napper, D.H. Steric stabilization in worse than θ-solvents. Colloid Polym. Sci. 1979, 257, 748–756. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V.; Priamitsyn, V.A. Theory of steric stabilization of colloid dispersions by grafted polymers. J. Colloid Interface Sci. 1990, 137, 495–511. [Google Scholar] [CrossRef]
- Gerber, P.; Moore, M.A. Comments on the Theory of Steric Stabilization. Macromolecules 1977, 10, 476–481. [Google Scholar] [CrossRef]
- Hamaker, H.C. The London—Van der Waals attraction between spherical particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Bergstr, L. Hamaker constants of inorganic materials. Adv. Colloid Interface Sci. 1997, 70, 125–169. [Google Scholar] [CrossRef]
- Srivastava, S.N.; Haydon, D.A. Estimate of the Hamaker constant for paraffinic hydrocarbons in aqueous suspensions. Trans. Faraday Soc. 1964, 60, 971. [Google Scholar] [CrossRef]
- Vial, J.; Carré, A. Calculation of Hamaker constant and surface energy of polymers by a simple group contribution method. Int. J. Adhes. Adhes. 1991, 11, 140–143. [Google Scholar] [CrossRef]
- Croucher, M.D.; Hair, M.L. Hamaker constants and the principle of corresponding states. J. Phys. Chem. 1977, 81, 1631–1636. [Google Scholar] [CrossRef]
- Visser, J. On Hamaker constants: A comparison between Hamaker constants and Lifshitz-van der Waals constants. Adv. Colloid Interface Sci. 1972, 3, 331–363. [Google Scholar] [CrossRef]
- Pinchuk, A.O. Size-Dependent Hamaker Constant for Silver Nanoparticles. J. Phys. Chem. C 2012, 116, 20099–20102. [Google Scholar] [CrossRef]
- Jiang, K.; Pinchuk, P. Temperature and size-dependent Hamaker constants for metal nanoparticles. Nanotechnology 2016, 27, 345710. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Kataby, G.; Cojocaru, M.; Prozorov, R.; Gedanken, A. Coating Carboxylic Acids on Amorphous Iron Nanoparticles. Langmuir 1999, 15, 1703–1708. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Voronin, E.F.; Pakhlov, E.M.; Zarko, V.I.; Turov, V.V.; Guzenko, N.V.; Lebod, R.; Chibowski, E. Features of fumed silica coverage with silanes having three or two groups reacting with the surface. Colloids Surf. Physicochem. Eng. Asp. 2000, 166, 187–201. [Google Scholar] [CrossRef]
- Ishida, H.; Koenig, J.L. Fourier transform infrared spectroscopic study of the structure of silane coupling agent on E-glass fiber. J. Colloid Interface Sci. 1978, 64, 565–576. [Google Scholar] [CrossRef]
- Murat, M.; Grest, G.S. Structure of a grafted polymer brush: A molecular dynamics simulation. Macromolecules 1989, 22, 4054–4059. [Google Scholar] [CrossRef]










| Particle | Size (nm) | Media | Disperse Method * | Stability | Stable Time |
|---|---|---|---|---|---|
| Oxides | |||||
| TiO2 | 2 | liquid paraffin | SSM. | stable | 1 month [10] |
| 5 | liquid paraffin | SSM. | not reported [33] | ||
| 10 | liquid paraffin | SSM. | stable | Claimed [34] | |
| 15 | PAO Spectrasyn 4 | SI-ATRP | stable | 56 days [11] | |
| 23 | PAO | None | aggregated [35] | ||
| 25 | liquid paraffin | Silanization + Dispersant | stable | 5 months [12] | |
| 75 | AOI group III | None | not reported [36] | ||
| SiO2 | 12 | PAO | NIM | stable | 2 days [13] |
| 15 | Ionic liquid | None | aggregated [37] | ||
| 23 | PAO Spectrasyn 4 | SI-ATRP | stable | 56 days [11] | |
| 23 | PAO | SI-ATRP | stable | 60 days [14,38] | |
| 25 | GMO | Silanization | stable | 5 months [39] | |
| 45 | EOT5 | None | not reported [15] | ||
| 60 | PAO 100 | Silanization | stable | 2 months [16] | |
| 100 | RO base oil | Silanization | stable | 8 days [17] | |
| 110 | PAO | Silanization | stable | 2 months [40] | |
| 200 | PAO | Silanization | stable | 4 months [18] | |
| 200 | PAO | Silanization | stable | 2 months [41] | |
| ZrO2/SiO2 | 100 | 20# machine oil | Other | stable | 12 h [42] |
| Al2O3/SiO2 | 70 | liquid paraffin | Silanization | stable | 3 months [19] |
| CuO | 5 | PAO | SSM. | stable | 30 days [43] |
| 40 | PAO 6 | None | not reported [44] | ||
| ZnO/CuO | 40 | vegetable oil | None | not reported [45] | |
| ZrO2 | 25 | PAO 6 | None | not reported [44] | |
| ZnO | 20 | PAO 6 | None | not reported [44] | |
| ZnO/Al2O3 | 50 | 20# machine oil | SSM. | stable | 28 days [20] |
| Fe3O4 | 10 | liquid paraffin | SSM. | stable | Claimed [46] |
| Fe2O3 | 30 | 500 SN basic oil | Dispersant | Stable | Claimed [47] |
| Al2O3 | 80 | 20# machine oil | SSM. | stable | 20 days [21] |
| Al2O3/TiO2 | 100 | base oil | Silanization | stable | 110 h [48] |
| GO/ZrO2 | 5 | liquid paraffin | Other | stable | 48 h [22] |
| Sulfide | |||||
| PbS | 5 | liquid paraffin | SSM. | stable | Claimed [49] |
| IF-WS2 | 65 | PAO | None | not reported [50] | |
| 100 | PAO | None | stable | hours [51] | |
| 110 | liquid paraffin | Silanization | stable | 14 days [23] | |
| 120 | liquid paraffin | None | aggregated [52] | ||
| WS2 | 7 | PAO | SSM. | stable | 6 months [24] |
| 100 | liquid paraffin | SSM. | stable | Claimed [25] | |
| 200 | liquid paraffin | SSM. | stable | 1 day [53] | |
| IF-MoS2 | 35 | PAO/Hexane | None | not reported [54] | |
| MoS2 | 3 | PAG | SSM. | stable | 2 weeks [55] |
| 15 | 250X base oil | Dispersant | stable | Claimed [56] | |
| 85 | EOT5 | None | not reported [15] | ||
| 90 | coconut/paraffin oil | SSM. | stable | 2 days [57] | |
| MoS2 sheets | N/A | PAO | SSM | stable | 7 days [58] |
| Mo15S19 | 10 | Linseed Oil | None | not reported [59] | |
| MoS2/TiO2 | 125 | rapeseed oil | None | stable | 2 days [26] |
| CuS | 20 | liquid paraffin | SSM. | stable | Claimed [60] |
| Metal | |||||
| Cu | 5 | liquid paraffin | SSM. | stable | months [10] |
| 9 | 500SN base oil | SSM. | stable | Claimed [61] | |
| 15 | liquid paraffin | SSM. | stable | Claimed [62] | |
| 40 | raw oil | Dispersant | not reported [63] | ||
| 75 | 500SN base oil | Dispersant | aggregated [64] | ||
| Carbon-coated Cu | 65 | PAO6 | None | not reported [65] | |
| Mo | 60 | PEG | None | not reported [66] | |
| Ni | 8 | PAO | SSM. | stable | 1 month [67] |
| 20 | 500SN base oil | Dispersant | stable | Claimed [68] | |
| 20 | PAO | None | stable | hours [69] | |
| Ag | 4 | liquid paraffin | SSM. | stable | months [10] |
| 4 | PAO | SSM. | stable | 3 months [70] | |
| 6 | PEG | SSM. | stable | 7 months [71] | |
| 6 | Kerosene | SSM. | stable | months [72] | |
| 10 | liquid paraffin | SSM. | not reported [73] | ||
| 15 | liquid paraffin | SSM. | stable | 7 days [74] | |
| Pd | 2 | liquid paraffin | SSM. | stable | Claimed [75] |
| 2 | liquid paraffin | SSM. | not reported [76] | ||
| 3 | PAO | SSM. | stable | months [77] | |
| Pb | 40 | liquid paraffin | SSM. | stable | claimed [78] |
| Carbon | |||||
| Graphene | N/A | liquid paraffin | Dispersant | not reported [79] | |
| N/A | SAE 10W30 | None | not reported [80] | ||
| N/A | PAO 9 | SSM. | stable | Claimed [81] | |
| N/A | SN350 | SSM. | stable | 6 h [29] | |
| N/A | hexadecane | SSM. | stable | 2 days [82] | |
| N/A | PEG400 | NIM | stable | 30 days [83] | |
| Graphene Oxide | N/A | mineral oil | Dispersant | not reported [84] | |
| N/A | SAE 5W30 | None | not reported [85] | ||
| N/A | PAO | None | not reported [86] | ||
| Carbon Spheres | 200 | SAE 5W30 | None | not reported [87] | |
| Nanodiamond | 5 | paraffin | None | not reported [88] | |
| 10 | paraffin | SSM | not reported [89] | ||
| 30 | Diisodecy Adipate | None | not reported [90] | ||
| 10 | ISO68 base oil | None | not reported [91] | ||
| Fullerene | 10 | mineral oil | None | not reported [92] | |
| Carbon nano-onions | 4 | PAO | None | aggregated [93] | |
| 10 | PAO | None | not reported [94] | ||
| 5 | PAO | None | not reported [95] | ||
| Carbon nano-horns | 97 | Mobil Pegasus 1005 | Dispersant | stable | 14 days [27] |
| Carbon nanotube | 100 | PAO | Dispersant | stable | 24 h [96] |
| N/A | lubricant oil | Dispersant | not reported [97] | ||
| N/A | liquid paraffin | None | not reported [98] | ||
| N/A | sunflower oil | Dispersant | not reported [99] | ||
| N/A | ionic liquid | None | stable | Claimed [100] | |
| N/A | rapeseed oil | SSM | stable | 10 days [101] | |
| Other | |||||
| CaCO3 | 20 | 500SN base oil | Dispersant | not reported [102] | |
| 40 | PAO | SSM. | stable | Claimed [103] | |
| 45 | dodecane /decane | Dispersant | stable | Claimed [104] | |
| Zinc borate | 35 | 500 SN basic oil | Dispersant | stable | Claimed [105] |
| ZnAl2O4 | 95 | Lubricant oil | SSM. | unstable [106] | |
| La(OH)3 | 30 | liquid paraffin | None | Not reported [107] | |
| LaB | 30 | 500SN base oil | Dispersant | stable | Claimed [108] |
| LaF3 | 6 | liquid paraffin | SSM. | stable | Claimed [109] |
| 8 | liquid paraffin | SSM. | stable | months [10] | |
| BN | 70 | POE | Silanization | stable | 10 days [110] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Renner, P.; Liang, H. Dispersion of Nanoparticles in Lubricating Oil: A Critical Review. Lubricants 2019, 7, 7. https://doi.org/10.3390/lubricants7010007
Chen Y, Renner P, Liang H. Dispersion of Nanoparticles in Lubricating Oil: A Critical Review. Lubricants. 2019; 7(1):7. https://doi.org/10.3390/lubricants7010007
Chicago/Turabian StyleChen, Yan, Peter Renner, and Hong Liang. 2019. "Dispersion of Nanoparticles in Lubricating Oil: A Critical Review" Lubricants 7, no. 1: 7. https://doi.org/10.3390/lubricants7010007