1. Introduction
Recently, photocatalytic composites have attracted much attention due to their applications potential in the environmental fields. Among of various composites, the carbon-based composites displayed the advantages of stable, highly electroconductive, and easy to be modified [
1]. Until now, there are a few reports on the preparation of the carbon-based composites, including carbon/tungsten acid bismuth [
2], carbon/iron and manganese oxides [
3], carbon/zinc oxide [
4], carbon/graphitic carbon nitride [
5], carbon-based quantum dots [
6], etc.
It is well known that TiO
2 is one of semiconductor photocatalysts with suitable band gap, high biological stability, and excellent photocatalytic properties [
7,
8]. It is well known that TiO
2 has a wide band-gap of 3.2 eV in the ultraviolet light zone, limiting its applications in the visible light band. Many strategies are applied to change the light absorption ability of TiO
2, such as dye sensitizing [
9], ion doping [
10], heterojunction [
11], etc. It is reported that the C-doped TiO
2 displayed the increase photocatalytic activity in the visible light region [
12]. Mohamed et al. synthesized the C-TiO
2 thin films via the sol-gel method using the Tween 80 both as the directing agent and the carbon source [
12]. Wu et al. prepared the C-TiO
2 composites with the narrow band gap and the optical property by the solvothermal method combining the calcination [
7]. Yao et al. used different calcination temperatures to prepare the C-doped TiO
2 nanoparticles with the high photocatalytic performance at 400 °C [
13]. Zhang et al. explored in situ create the C-doped hollow TiO
2 spheres with a superior photocatalytic activity [
14].
Bamboo is one of typical biomass, which can be used as a carbon source. Bamboo-based carbon materials have wide applications due to their strong adsorption ability for organic pollutants and metal ions [
15]. Bamboo charcoal is an economical carbon during the preparation procedure, when compared with the other carbon precursors (graphene, carbon nanotubes, and fullerene) [
16]. Chou et al. prepare the bamboo charcoal/TiO
2 composites in a high-efficiency dye sensitized solar cell [
17,
18]. Vignesh et al. synthesized the bamboo charcoal/TiO
2 composite with plasma surface treatment by the sol-gel method [
19]. Recently, Qian et al. produced the biomorphic charcoal/TiO
2 composites using the moso bamboo as templates [
20]. However, as far as we know, there has been little report on the synthesis of the bamboo charcoal/TiO
2 composites in the environmental field.
In this article, the bamboo charcoal/TiO2 composites were synthesized by the calcination route using the C16H36O4Ti as the titanium source and the bamboo powder as the carbon source. The synergistic effects of carbon doping with TiO2 were explored in detail. The photocatalytic activities of the bamboo charcoal/TiO2 composites were investigated under the visible light irradiation and UV irradiation.
3. Results and Discussion
The thermal behavior of the precursors was investigated with TG and DTG under nitrogen atmosphere. As shown in
Figure 2A, all of the samples contained about 2.7% of absorbed water (the weight loss below 100 °C), and the total weight losses of the bamboo power composites were 65.5%, 65.8%, 68.3%, and 56.5% for P-S1, P-S2, P-S3, and P-S4, respectively. These results indicated that with the decreased size of bamboo powders, the total weight losses slightly increased first then decreased. The initially degradation temperature was 240 °C for all of the samples. For the samples of P-S1, P-S2, and P-S3, the weight loss was mainly attributed to the transformation of bamboo power to carbon (
Figure 2B), according with the endothermic peak located at 310 °C. The endothermic peak for P-S4 located at 265 °C. In general, the carbon-based samples showed the maximum weight loss around 580 °C. In this article, one can observe a loss peak 265 °C due to the existence of cellulose, hemicellulose, and lignin. These results indicated that the samples that were prepared using bamboo power with different sizes had different thermal stabilities.
SEM measurements were applied to characterize the microstructures and morphologies of the precursors. Bamboo powder has a two-dimensional (2D) structure and many thin wrinkles were dispersed on the surface (
Figure 3(P-S0)). In the previous study, these wrinkles were reported to induce the form of nano-channels on the surface of bamboo powder materials, which could transport electrons to suppress the recombination of photo-excited electron-hole pairs [
21]. When the bamboo powder was used as the matrix, one can see that a large amount of TiO
2 particles were dispersed on the surfaces of bamboo powder, as shown in
Figure 3(P-S1 to P-S4). It was observed that the TiO
2 particles were aggregated to form an irregular structure (
Figure 3(P-S1 to P-S3)). When the size of the bamboo powder was reduced to 0.096 mm, one can see that the quantity of uniform TiO
2 particles dramatically increased (
Figure 3(P-S4)).
SEM measurements were also conducted to characterize the microstructures and the morphologies of the samples after calcination. One can find that, after calcining, the surface of the bamboo charcoal still existed thin wrinkles and generated the small pore structure (
Figure 4(C-S0)). As shown in
Figure 4(C-S1), TiO
2 particles were dispersed on the surface of bamboo charcoal. The mean size of TiO
2 particles generated in the experiments is 408.6 nm, and the aperture of bamboo charcoal are mainly concentrated in about 2.27 μm (the size distribution of C-S1). Pore diameter of bamboo charcoal is about five times as much as particle size of TiO
2 particles. As shown in both
Figure 4(C-S2) and
Figure 4(C-S3), TiO
2 particles were intensively distributed on the surface and the edge of macroporous in the bamboo charcoal, which piled into irregular structure and did not block the pore structure of bamboo charcoal. But the quantity of TiO
2 particles remarkably decreased. Based on these results, one can conclude that the size of bamboo had influence on the morphology of bamboo charcoal/TiO
2 composites.
FT-IR spectra were conducted to study the functional groups of the as-obtained samples. The FT-IR spectra of precursors of bamboo charcoal-based composites, which were prepared at room temperature for 1 h, were shown in
Figure 5A. It observed the characteristic peaks of cellulose, hemicellulose, and lignin. The stretching vibration in OH group of hemicellulose was observed at ~3410 cm
−1. The characteristic peaks of hemicellulose were also observed at ~1450, 1160, and 1034 cm
−1 in the spectrum. The shoulder at ~1160 cm
−1 belongs to the arabinosyl side chains. The characteristic peak of all the C-O, C-C stretching, and the glycosidic (C-O-C) located at ~1034 cm
−1 [
22]. In the literature, it obtained the characteristic peak of the C-O stretching mode of cellulose at ~1034 cm
−1 [
23]. The peak at ~2897 cm
−1 is due to the C-H stretching in CH
2 and CH
3 groups of cellulose, which is unaffected by changes of crystallinity. The peaks at ~1503 and ~1595 cm
−1 indicated the aromatic skeletal vibration of lignin. It observed the absorbance peak of Ti-O that was stretching at 758 cm
−1 [
24].
The bamboo charcoal/TiO
2 composites were obtained using the precursors by calcinated at 800 °C for 3 h under the flowing nitrogen gas.
Figure 5B showed the FT-IR spectra of as-obtained bamboo charcoal/TiO
2 composites samples. In comparison with
Figure 5A, it observed the dramatically decreased intensities of peaks at 3410, 2897, and 1595 cm
−1, thus demonstrating the degraded bamboo powder during the calcining process. In addition, it observed the absorption band of Ti-O stretching at 817 cm
−1 for the bamboo charcoal/TiO
2 composites, implying the as-fabricated bamboo charcoal/TiO
2 composites by this method.
X-ray analysis provides the information about the crystal phase of the as-obtained materials. In the literature, it reported the greatly affected photocatalytic activity by the phase and crystallinity of the catalysts [
12].
Figure 6(C-S0) displayed the XRD pattern of bamboo charcoal composites without adding tetrabutyl titanate. One can clearly observe a characteristic diffraction peak of cellulose. It is interesting to find that the bamboo charcoal/TiO
2 composites are composed of the mixed phases of anatase and rutile, as shown in
Figure 6(C-S1) and
Figure 6(P-S1). Tetrabutyl titanate and the water reacted and fabricated TiO
2 crystal nucleus by hydrolysis and the condensation reaction in this system. Reaction equations are listed, as follows:
In order to reduce the free energy of the particle in the surface, TiO
2 crystal nucleus tend to spontaneously reunite to form the aggregate, which gradually grew up because of Gibbs-Thomson effect. Eventually, TiO
2 nanoparticles formed. In the XRD pattern of the precursor P-S1, it observed the six peaks of TiO
2 at 2θ = 25.35, 37.91, 48.05, 62.76, 68.43, and 74.05, indexed to the (101), (004), (200), (204), (116), and (107) planes of TiO
2, respectively (JCPDS No. 99-0008). It observed three peaks of TiO
2 at 2θ = 28.19, 40.90, and 54.37, which were indexed to the (110), (111), and (211) planes of the rutile phase of TiO
2. Calcinated the precursors at 800 °C for 3 h under the flowing nitrogen gas, the peaks intensities of TiO
2 were obviously increased, implying the increase crystallinity of TiO
2. In the literature, it reported the phase transformation of anatase to rutile at around 600 °C [
25]. In this article, 800 °C was chosen as the calcination temperature. The crystallinity of the bamboo charcoal/TiO
2 composites was 93.2%, which is enhanced remarkably, when compared with that of precursor (78.3%).
Figure 7A is UV-visible absorption spectrum of bamboo charcoal-based composites precursors with different sizes. For all of the samples, the absorption is significantly enhanced at a wavelength less than 400 nm region due to TiO
2 intrinsic absorption band (3.2 eV). With the decreasing of particle sizes, peak intensity gradually increased, and the absorption peak moved towards long wave, which was conducive to the absorption of visible light.
Figure 7B is photocatalytic degradation of MB under UV irradiation by the bamboo charcoal-based composites precursors. One can easily see that with the decreasing of particle sizes, photocatalytic degradation rate of MB gradually enhanced, indicating that particle sizes played an important role in the photocatalytic activity.
The adsorption experiment was performed to evaluate the adsorption ability of C-S1 and C-S0 in the dark by UV-vis spectrum. The adsorption rate curves of MB on the composites were investigated, as shown in
Figure 8. After equilibration for 60 min, 30.7% of MB was removed from the solution C-S1 and 9.7% of MB was removed from the solution of C-S0, respectively. For the sample of C-S0, there was a rapid adsorption within the first 10 min, it slowly leveled off, and then reached adsorption equilibrium in 30 min. For the sample of C-S1, there was a rapid adsorption within the first 30 min, then slowly leveled off, and reached to adsorption equilibrium in 60 min. When compared with the sample of C-S0, the adsorption ability of MB in the CS1 composite increased due to the increase of specific surface area (
Table 1). These results indicated that the as-obtained composites displayed the enhanced absorptive capacity.
The equilibrium adsorption about MB was conducted using the adsorption isotherms, such as Langmuir and Freundlich isotherm models. It reported a homogeneous system of the Langmuir isotherm and a heterogeneous system of Freundlich isotherm [
23].
Figure 9 displayed the adsorption data. It found little high correlation coefficients for the Freundlich isotherm model (R
2 = 0.988, 0.989), when compared with that for the Langmuir isotherm model (R
2 = 0.952, 0.957).
By measuring the decomposition rate of MB in water under both ultraviolet light and visible light irradiation, it evaluated the photocatalytic activity of bamboo charcoal/TiO
2 composites.
Figure 10A displayed the photocatalytic activity of as-obtained composites with different sizes under the UV irradiation. The sample without catalysts was used as control (
Figure 10A). Bamboo charcoal/TiO
2 composites showed higher catalytic activity than that without TiO
2. After the UV irradiation for 60 min, the degradation rates were 95%, 87%, 49%, and 86% for C-S1, C-S2, C-S3, and C-S4, respectively. The nitrogen adsorption-desorption isotherms results for the samples are listed in
Table 1. It was found that the surface area decreased with the decreasing sizes of bamboo charcoal. The pore volume also presented a similar result. Both the porous structure and the high surface area are beneficial for reactant adsorption. Therefore, with the decrease size of bamboo charcoal, photocatalytic degradation efficiency decreased. When the size is less than 0.096 nm, the photodegradation efficiency gradually improved.
Figure 10B exhibited the photocatalytic activity of as-obtained composites with different sizes under visible light irradiation. In the visible light catalytic after 60 min, the degradation rates were 97%, 91%, 40%, and 78% for C-S1, C-S2, C-S3, and C-S4, respectively. Photocatalytic activity of bamboo charcoal/TiO
2 decreased first then increased with the decreasing size of bamboo charcoal.
It observed the photocatalytic reaction following pseudo-first-order kinetics ln(C
0/C) = kt using MB with low concentration, where C
0 is the initial concentration of MB solution after adsorption equilibrium, C is the MB concentration at any time t, k is on behalf of the kinetic constant. The equation was applied to simulate the light catalytic data, explaining the reaction kinetics of the MB degradation. Under the UV irradiation, it observed the relative coefficient R
2 of the fitting curves of 0.976, 0.963, 0.992, 0.983, 0.987, and 0.896 for C-S1, C-S2, C-S3, C-S4, C-S0, and MB, respectively. The values are 0.991, 1.000, 0.968, 0.987, 0.857, and 0.798 for C-S1, C-S2, C-S3, C-S4, C-S0, and MB under the visible light irradiation. Before the UV irradiation, it observed the value of total organic carbon (TOC) of 19.32 mg L
−1 for MB. After the UV irradiation, it observed the decrease value of TOC with the charcoal/TiO
2 nanocomposites. Therefore, one can conclude the photodegradation reactions following the pseudo-first-order kinetics (
Figure 11). Meanwhile, the kinetic constants decreased first, then increased with decreasing bamboo charcoal sizes. These results indicated that the photocatalytic activity of bamboo charcoal/TiO
2 composites decreased first then increased with the decreasing size of bamboo charcoal.
In order to examine the stability of photocatalytic performance of bamboo charcoal/TiO
2 composites, repeatability test of the sample was done.
Figure 12a,b displayed the absorption spectra of MB solution under the visible light irradiation in the presence of C-S1 in the first and second cycle. One can see that with the increase of time, maximum absorbance of MB decrease constantly. In the second circulation, the degradation rate of MB almost unchanged, when compared with that of the first time.
Figure 12c is photocatalytic degradation of MB after up to four cycles measured after the visible light irradiation of duration 240 min. After the bamboo charcoal/TiO
2 composites were used for four times, the photocatalytic degradation rate was 75%. While the degradation rate kept stability as compared with the first cycle (97%). Further study showed that the bamboo charcoal/TiO
2 composites are easy to be removed and re-used many times.
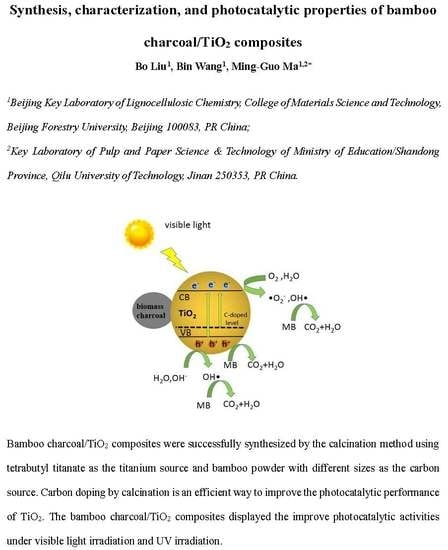
Based on the above study, one speculates the feasible mechanism of photocatalytic degradation over bamboo charcoal/TiO
2 composites, as following: In the bamboo charcoal/TiO
2 composites, bamboo charcoal itself displayed no catalytic effect, which was used matrix for the synthesis of bamboo charcoal/TiO
2 composites with large specific surface area. These structures are advantageous for the improve photocatalytic reaction on the bamboo charcoal/TiO
2 composites. As shown in the
Figure 13, under visible light irradiation, the conduction band and valence band of TiO
2 generate, respectively, electrons and holes; the photoproduction hole of valence band has a strong oxidizing. It can put the water and hydroxyl oxidatize into hydroxyl radicals. The generated hydroxyl radicals can also oxidatize and decompose pollutants. At the same time, electronics of conduction band can react with oxygen and water to generate hydroxyl free radicals and the superoxide free radicals and other strong oxidizing species. •O
2−, OH•, and holes decompose pollutants into CO
2 and H
2O. Bamboo charcoal also reinforced adsorption the ability of composites to MB, promoting the proceed of photocatalytic reaction on the surface of composites.