TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2-ZnO Oxide Systems Using the Sol-Gel Method
2.3. Analysis of Materials
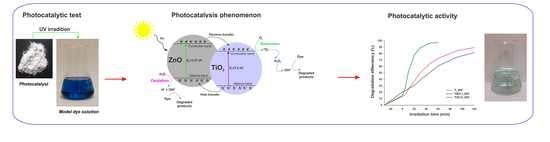
2.4. Photocatalytic Tests
2.5. Kinetic Study
3. Results and Discussion
3.1. Dispersive and Morphological Characteristics
3.2. Structural Characteristics
3.3. Surface Composition
3.4. Porous Structure Parameters
3.5. FTIR Analysis
3.6. Thermal Analysis
3.7. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gross, S.; Muller, K. Sol-gel derived silica-based organic-inorganic hybrid materials as composite precursors for the synthesis of highly homogeneous nanostructured mixed oxides: An overview. J. Sol-Gel Sci. Technol. 2011, 60, 283–298. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Aggrawal, S.; Mohanty, C.; Mohanty, P. Metal oxide nanostructures incorporated/immobilized paper matrices and their applications: A review. RSC Adv. 2015, 5, 83036–83055. [Google Scholar] [CrossRef]
- Debecker, D.P.; Hulea, V.; Mutin, P.H. Mesoporous mixed oxide catalysts via non-hydrolytic sol-gel: A review. Appl. Catal. A 2013, 451, 192–206. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Mir, N.A.; Qutub, N.; Mehraj, O.; Sabir, S.; Muneer, M. Synthesis, characterization and optimization of photocatalytic activity of TiO2/ZrO2 nanocomposite heterostructures. Mater. Sci. Eng. B 2015, 193, 137–145. [Google Scholar] [CrossRef]
- Du, X.; Men, K.; Xu, Y.; Li, B.; Yang, Z.; Liu, Z.; Li, L.; Li, L.; Feng, T.; ur Rehman, W.; et al. Enhanced capacitance perfomance of Al2O3-TiO2 composite thin film via sol-gel using double chelators. J. Colloid Interface Sci. 2015, 443, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, Y.; Suo, H.; Qing, M.; Yan, L.; Wu, B.; Xu, J.; Xiang, H.; Li, Y. Effects of ZrO2 promoter on physic-chemical properties and activity of Co/TiO2-SiO2 Fischer-Tropsch catalysts. J. Mol. Catal. A Chem. 2015, 396, 108–119. [Google Scholar] [CrossRef]
- Michalow, K.A.; Otal, E.H.; Burnat, D.; Fortunato, G.; Emerich, H.; Ferri, D.; Heel, A.; Graule, T. Flame-made visible light active TiO2:Cr photocatalysts: Correlation between structural, optical and photocatalytic properties. Catal. Today 2013, 209, 47–53. [Google Scholar] [CrossRef]
- Pouretedal, H.R. Visible photocatalytic activity of co-doped TiO2/Zr,N nanoparticles in wastewater treatment of nitrotoluene samples. J. Alloys Compd. 2018, 735, 2507–2511. [Google Scholar] [CrossRef]
- Park, J.-H.; Jang, I.; Song, K.; Oh, S.-G. Surfactants-assisted preparation of TiO2-Mn oxide composites and their catalytic activities for degradation of organic pollutant. J. Phys. Chem. Solids 2013, 74, 1056–1062. [Google Scholar] [CrossRef]
- Cui, M.; Pan, S.; Tang, Z.; Chen, X.; Qiao, X.; Xu, Q. Physiochemical properties of n-n heterostructured TiO2/Mo-TiO2 composites and their photocatalytic degradation of gaseous toluene. Chem. Speciat. Bioavailab. 2017, 29, 60–69. [Google Scholar] [CrossRef]
- Garmaroudi, Z.A.; Mohammadi, M.R. Design of TiO2 dye-sensitized solar cell photoanode electrodes with different microstructures and arrangement modes of the layers. J. Sol-Gel Sci. Technol. 2015, 76, 666–678. [Google Scholar] [CrossRef]
- Barea, E.M.; Bisquert, J. Properties of chromophores determining recombination at the TiO2-dye-electrolyte interface. Langmuir 2013, 29, 8773–8781. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Liu, W.B.; Fu, P.; Cheng, W.L. Enhanced photoactivity of N-doped TiO2 for Cr(VI) removal: Influencing factors and mechanism. Korean J. Chem. Eng. 2017, 34, 1584–1590. [Google Scholar] [CrossRef]
- He, Y.; Fu, Z.; Zhou, Q.; Zhong, M.; Yuan, L.; Wei, J.; Yang, X.; Wang, C.; Zeng, Y. Fabrication and electrochemical behavior of a lithium-sulfur cell with a TiO2-sulfur-carbon aerogel-based cathode. Ionics 2015, 21, 3065–3073. [Google Scholar] [CrossRef]
- Orge, C.A.; Faria, J.L.; Pereira, M.F.R. Photocatalytic ozonation of aniline with TiO2-carbon composite materials. J. Environ. Manag. 2017, 195, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Delsouz Khaki, M.R.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Soltan, S.; Jafari, H.; Afshar, S.; Zabihi, O. Enhancement of photocatalytic degradation of furfural and acetophenone in water media using nano-TiO2-SiO2 deposited on cementitious materials. Water Sci. Technol. 2016, 74, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Sobhanardakani, S.; Zandipak, R. Synthesis and application of TiO2/SiO2/Fe3O4 nanoparticles as novel adsorbent for removal of Cd(II), Hg(II) and Ni(II) ions from water samples. Clean Technol. Environ. 2017, 19, 1913–1925. [Google Scholar] [CrossRef]
- Stoyanova, A.; Hitkova, H.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Ivanova, M.; Sredkova, N. Synthesis and antibacterial activity of TiO2/ZnO nanocomposites prepared via nonhydrolytic route. J. Chem. Technol. Metall. 2013, 48, 154–161. [Google Scholar]
- Fan, M.; Hu, S.; Ren, B.; Wang, J.; Jing, X. Synthesis of nanocomposite TiO2/ZrO2 prepared by different templates and photocatalytic properties for the photodegradation of Rhodamine B. Powder Technol. 2013, 235, 27–32. [Google Scholar] [CrossRef]
- Duraisamy, N.; Thangavelu, R.R. Synthesis, characterization and photocatalytic properties of TiO2-SnO2 composite nanoparticles. Adv. Mater. Res. 2013, 678, 373–377. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Nolan, M.; Iwaszuk, A.; Lucid, A.K.; Carey, J.J.; Fronzi, M. Design of novel visible light active photocatalyst materials: Surface modified TiO2. Adv. Mater. 2016, 28, 5425–5446. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, Q.; Luan, W.L. Preparation and electric properties of dense nanocrystalline zinc oxide ceramics. J. Am. Ceram. Soc. 2002, 85, 1016–1018. [Google Scholar] [CrossRef]
- Ulyankina, A.; Leontyev, I.; Avramenko, M.; Zhigunov, D.; Smirnova, N. Large-scale synthesis of ZnO nanostructures by pulse electrochemical method and their photocatalytic properties. Mater. Sci. Semicond. Process. 2018, 76, 7–13. [Google Scholar] [CrossRef]
- Tian, J.; Chen, L.; Dai, J. Preparation and characterization of TiO2, ZnO, and TiO2/ZnO nanofilms via sol-gel process. Ceram. Int. 2009, 35, 2261–2270. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Wang, Y.; Kang, Y.; Wang, C. Fabrication of TiO2/ZnO composite nanofibers with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 7834–7838. [Google Scholar] [CrossRef]
- Arabnezhad, M.; Afarani, M.S.; Jafari, A. Co-precipitation synthesis of ZnO-TiO2 nanostructure composites for arsenic photodegradation from industrial wastewater. Int. J. Environ. Sci. Technol. 2017, 1–6. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Bezverkhyy, I.; Skompskab, M. ZnO nanorods covered with a TiO2 layer: Simple sol-gel preparation, and optical, photocatalytic and photoelectrochemical properties. J. Mater. Chem. A 2015, 3, 12748–12760. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.; Yu, Y.; Zhang, P.; Yuan, J.; Cao, Y.; Cao, Y.; Xu, J. Investigation on a novel ZnO/TiO2-B photocatalyst with enhanced visible photocatalytic activity. Physica E 2014, 58, 118–123. [Google Scholar] [CrossRef]
- Vlazan, P.; Ursu, D.H.; Irina-Moisescu, C.; Mirona, I.; Sfirloaga, P.; Rusu, E. Structural and electrical properties of TiO2/ZnO core-shell nanoparticles synthesized by hydrothermal method. Mater. Charact. 2015, 101, 153–158. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A highly efficient TiO2@ZnO n–p–n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G. Novel nanocomposite hydrogels consisting of layered double hydroxide with ultrahigh tensibility and hierarchical porous structure at low inorganic content. Adv. Mater. 2014, 26, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; El-Salamony, R.A. Photocatalytic disc-shaped composite systems for removal of hazardous dyes in aqueous solutions. Can. Chem. Trans. 2014, 2, 56–70. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mi, W.; Tian, J.; Dai, J.; Wang, X.; Liu, X. Effect of calcinations of TiO2/ZnO composite powder at high temperature on photodegradation of methyl orange. Compos. Part B Eng. 2013, 45, 758–767. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chang, Y.Y.; Huang, H.L.; Hsu, J.-T.; Chen, Y.-C.; Wu, A.Y.-J. Characterization and antibacterial performance of bioactive Ti-Zn-O coatings deposited on titanium implants. Thin Solid Films 2013, 528, 143–150. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, K.A.; Khan, W.U. ZnO/TiO2 nanocomposite synthesized by sol gel from highly soluble single source molecular precursor. Chin. J. Chem. Phys. 2014, 27, 548–554. [Google Scholar] [CrossRef]
- Perez-Larios, A.; Lopez, R.; Hernandez-Gordillo, A.; Tzompantzi, F.; Gomez, R.; Torres-Guerra, L.M. Improved hydrogen production from water splitting using TiO2-ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Morales-Luna, M.; Arvizua, M.A.; Tellez-Cruz, M.M. Optical, structural, and morphological properties of photocatalytic TiO2-ZnO thin films synthesized by the sol-gel process. Thin Solid Films 2015, 594, 304–309. [Google Scholar] [CrossRef]
- Shalaby, A.; Dimitriev, Y.; Iordanova, R.; Bachvarova-Nedelcheva, A.; Iliev, T. Modified sol-gel synthesis of submicron powders in the system ZnO-TiO2. J. Univ. Chem. Technol. Mater. 2011, 46, 137–142. [Google Scholar]
- Moradi, S.; Azar, P.A.; Farshid, S.R.; Khorrami, S.A.; Givianrad, M.H. Effect of additives on characterization and photocatalytic activity of TiO2/ZnO nanocomposite prepared via sol-gel process. Int. J. Chem. Eng. 2012, 2012, 215373. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Huang, W.; Yang, C.; Huang, T.; Situ, Y.; Huang, H. Synthesis of porous ZnO/TiO2 thin films with superhydrophilicity and photocatalytic activity via a template-free sol-gel method. Surf. Coat. Technol. 2014, 258, 531–538. [Google Scholar] [CrossRef]
- Tian, J.; Chen, L.; Yin, Y.; Wang, X.; Dai, J.; Zhu, Z.; Liu, X.; Wu, P. Photocatalyst of TiO2/ZnO nano composite film: Preparation, characterization, and photodegradation activity of methyl orange. Surf. Coat. Technol. 2009, 204, 205–214. [Google Scholar] [CrossRef]
- Naseri, N.; Yousefi, M.; Moshfegh, A.Z. A comparative study on photoelectrochemical activity of ZnO/TiO2 and TiO2/ZnO nanolayer systems under visible irradiation. Solar Energy 2011, 85, 1972–1978. [Google Scholar] [CrossRef]
- Prasannalakshmi, P.; Shanmugam, N. Fabrication of TiO2/ZnO nanocomposites for solar energy driven photocatalysis. Mater. Sci. Semicond. Process. 2017, 61, 114–124. [Google Scholar] [CrossRef]
- Mitchell, D.R.G. Circular Hough transform diffraction analysis: A software tool for automated measurement of selected area electron diffraction patterns within Digital Micrograph™. Ultramicroscopy 2008, 108, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Xie, Y.; Li, C.; Han, G.; Zhang, L.; Xu, R.; Zhang, X. Investigation on solar photocatalytic degradation of various dyes in the presence of Er3+:YAlO3/ZnO-TiO2 composite. J. Environ. Manag. 2010, 91, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-W.; Fu, Y.-S.; Sun, J.; Han, X.; Liu, J. Complete UV emission of ZnO nanoparticles in a PMMA matrix. Semicond. Sci. Technol. 2006, 21, 1202–1206. [Google Scholar] [CrossRef]
- Morsi, R.E.; Elsalamony, R.A. Superabsorbent enhanced-catalytic core/shell nanocomposites hydrogels for efficient water decolorization. New J. Chem. 2016, 1542, 33–36. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.C.; Zhang, L.; Wang, X.; Ho, W. Preparation of a highly active nanocrystalline TiO2 photocatalyst from titanium oxo cluster precursor. J. Solid State Chem. 2004, 177, 2584–2590. [Google Scholar] [CrossRef]
- Lotus, A.F.; Tacastacas, S.N.; Pinti, M.J.; Britton, L.A.; Stojilovic, N.; Ramsier, R.D.; Chase, G.G. Fabrication and characterization of TiO2-ZnO composite nanofibers. Physica E 2011, 43, 857–861. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshikawa, S. Synthesis and thermal analyses of TiO2-derived nanotubes prepared by the hydrothermal method. J. Mater. Res. 2004, 19, 982–985. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Zdarta, J.; Paukszta, D.; Jesionowski, T. The influence of addition of a catalyst and chelating agent on the properties of titanium dioxide synthesized via the sol-gel method. J. Sol-Gel Sci. Technol. 2015, 75, 264–278. [Google Scholar] [CrossRef]
- Das, S.; Meena, S.S.; Pramanik, A. Zinc oxide functionalized human hair: A potential water decontaminating agent. J. Colloid Interface Sci. 2016, 462, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fu, X.; Han, Y.; Chang, E.; Wu, H.; Wang, H.; Li, K.; Qi, X. Preparation, characterization, and photocatalytic activity of TiO2/ZnO nanocomposites. J. Nanomater. 2013, 2013, 321459. [Google Scholar] [CrossRef]
- Pei, C.C.; Leung, W.W.-F. Photocatalytic degradation of Rhodamine B by TiO2/ZnO nanofibers under visible-light irradiation. Separ. Purif. Technol. 2013, 114, 108–116. [Google Scholar] [CrossRef]
- Chen, J.D.; Liao, W.S.; Jiang, Y.; Yu, D.N.; Zou, M.L.; Zhu, H.; Zhang, M.; Du, M.L. Facile fabrication of ZnO/TiO2 heterogeneous nanofibres and their photocatalytic behaviour and mechanism towards Rhodamine B. Nanomater. Nanotechnol. 2016, 6, 9. [Google Scholar] [CrossRef]
- Araújo, E.S.; da Costa, B.P.; Oliveira, R.A.P.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. TiO2/ZnO hierarchical heteronanostructures: Synthesis, characterization and application as photocatalysts. J. Environ. Chem. Eng. 2016, 4, 2820–2829. [Google Scholar] [CrossRef]
- Agrawal, M.; Gupta, S.; Pich, A.; Zafeiropoulos, N.E.; Stamm, M. A facile approach to fabrication of ZnO-TiO2 hollow spheres. Chem. Mater. 2009, 21, 5343–5348. [Google Scholar] [CrossRef]
- Zhang, D. Effectiveness of photodecomposition of Rhodamine B and Malachite Green upon coupled tricomponent TiO2(Anatase-Rutile)/ZnO nanocomposite. Acta Chim. Slovaca 2013, 2, 245–255. [Google Scholar] [CrossRef]
- Rahman, M.M.; Roy, D.; Mukit, M.S.H. Investigation on the relative degradation of Methylene Blue (MB) and Rhodamine-B (RB) dyes under UV-Visible light using thermally treated commercial and doped TiO2/ZnO photocatalysts. Int. J. Integr. Sci. Technol. 2016, 2, 14–18. [Google Scholar]











| Color Index Name | C.I. Basic Blue 9 | C.I. Basic Red 1 | C.I. Basic Violet 10 |
|---|---|---|---|
| Chemical structure |  |  |  |
| Molecular formula | C16H18ClN3S·3H2O | C28H31ClN2O3 | C28H31ClN2O3 |
| Molar mass (g/mol) | 373.85 | 479.01 | 479.01 |
| λmax (nm) | 664 | 526 | 553 |
| Sample Name | Molar Ratio TiO2:ZnO | Temperature of Calcination (°C) | Particle Diameter Range (nm) | Dominant Particles Diameter with Maximum Volume Contribution (%) | Polydispersity Index |
|---|---|---|---|---|---|
| Ti | 10:0 | - | 531–1720 | 955 nm—21.6 | 0.183 |
| Ti9Zn1 | 9:1 | 459–1110 | 712 nm—27.5 | 0.312 | |
| Ti5Zn2 | 5:2 | 459–1480 | 825 nm—25.9 | 0.361 | |
| Ti1Zn3 | 1:3 | 396–825 | 615 nm—31.7 | 0.150 | |
| Zn | 0:10 | 220–615 | 396 nm—25.7 | 0.178 | |
| Ti_600 | 10:0 | 600 | 615–1990 | 1110 nm—23.8 | 0.220 |
| Ti9Zn1_600 | 9:1 | 531–1280 | 825 nm—32.6 | 0.408 | |
| Ti5Zn2_600 | 5:2 | 459–955 | 712 nm—32.2 | 0.504 | |
| Ti1Zn3_600 | 1:3 | 255–615 | 396 nm—26.7 | 0.182 | |
| Zn_600 | 0:10 | 51–122 220–1110 | 79 nm—5.1 531 nm—14.2 | 0.434 |
| Sample Name | Molar Ratio TiO2:ZnO | Temperature of Calcination (°C) | Specific Surface Area ABET (m2/g) | Total Pore Volume Vp (cm3/g) | Average Pore Size Sp (nm) |
|---|---|---|---|---|---|
| Ti | 10:0 | - | 488.6 | 0.046 | 1.9 |
| Ti9Zn1 | 9:1 | 494.7 | 0.079 | 1.9 | |
| Ti5Zn2 | 5:2 | 475.8 | 0.051 | 1.9 | |
| Ti1Zn3 | 1:3 | 97.0 | 0.030 | 2.0 | |
| Zn | 0:10 | 27.2 | 0.008 | 2.1 | |
| Ti_600 | 10:0 | 600 | 26.5 | 0.010 | 2.2 |
| Ti9Zn1_600 | 9:1 | 7.6 | 0.003 | 2.1 | |
| Ti5Zn2_600 | 5:2 | 7.2 | 0.005 | 2.2 | |
| Ti1Zn3_600 | 1:3 | 7.5 | 0.005 | 2.3 | |
| Zn_600 | 0:10 | 11.5 | 0.007 | 2.2 |
| Sample Name | Concentration of Dye Solution (mg/dm3) | Efficiency of Decomposition (%) | Ref. | ||
|---|---|---|---|---|---|
| C.I. Basic Blue 9 | C.I. Basic Red 1 | C.I. Basic Violet 10 | |||
| Ti9Zn1_600 | 5 | 97.2 | 93.6 | 93.4 | this work |
| (TiO2)1−x-(ZnO)x | 10 | 45.0–62.0 | - | - | [45] |
| TZO1-TZO4 | 1 | ~100 | - | - | [51] |
| TiO2 | 5 | 98.3 | - | - | [59] |
| TiO2/ZnO | 0.5 | - | - | ~100 | [62] |
| ZnO/TiO2 | 2 | - | - | 90.0 | [63] |
| TiO2/ZnO | 4.8 | - | - | 90.0 | [64] |
| TiO2/ZnO | 4.8 | - | ~100 | - | [65] |
| ZnO-TiO2 | 4.8 | - | - | ~100 | [66] |
| TiO2/ZnO | 20 | 96.0 | - | 83.0 | [67] |
| Sample Name | k (1/min) | R2 | t1/2 (min) |
|---|---|---|---|
| C.I. Basic Blue 9 | |||
| Ti_600 | 0.0186 | 0.9937 | 37.269 |
| Ti9Zn1_600 | 0.0596 | 0.9731 | 11.632 |
| Ti5Zn2_600 | 0.0141 | 0.9957 | 49.178 |
| C.I. Basic Red 1 | |||
| Ti_600 | 0.0172 | 0.9584 | 40.398 |
| Ti9Zn1_600 | 0.0459 | 0.9930 | 15.086 |
| Ti5Zn2_600 | 0.0074 | 0.9922 | 93.225 |
| C.I. Basic Violet 10 | |||
| Ti_600 | 0.0174 | 0.9918 | 39.727 |
| Ti9Zn1_600 | 0.0453 | 0.9897 | 15.301 |
| Ti5Zn2_600 | 0.0103 | 0.9765 | 67.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. https://doi.org/10.3390/ma11050841
Siwińska-Stefańska K, Kubiak A, Piasecki A, Goscianska J, Nowaczyk G, Jurga S, Jesionowski T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials. 2018; 11(5):841. https://doi.org/10.3390/ma11050841
Chicago/Turabian StyleSiwińska-Stefańska, Katarzyna, Adam Kubiak, Adam Piasecki, Joanna Goscianska, Grzegorz Nowaczyk, Stefan Jurga, and Teofil Jesionowski. 2018. "TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity" Materials 11, no. 5: 841. https://doi.org/10.3390/ma11050841