Dual Light- and pH-Responsive Composite of Polyazo-Derivative Grafted Cellulose Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Cellulose Nanocrystals
2.2.2. Immobilization of Initiator on Cellulose Nanocrystals
2.2.3. Grafting of MMAZO from Initiator-Functionalized Cellulose Nanocrystals
2.2.4. Preparation of PMMAZO-Grafted CNCs/PU Films
2.2.5. Cleavage of Polymer Brushes from PMMAZO-grafted CNCs
2.2.6. Measurement of the Responsive Behavior of PMMAZO-Grafted CNCs and Their Composite
2.2.7. TEM, FT-IR, NMR, XPS, and EA Analysis
2.2.8. XRD, TGA, UV–VIS Absorption Spectra and GPC
3. Results and Discussion
3.1. Cellulose Nanocrystals Grafted with PMMAZO
3.2. Properties of the PMMAZO-Grafted CNCs
3.2.1. Crystal Structure and Thermal Analysis
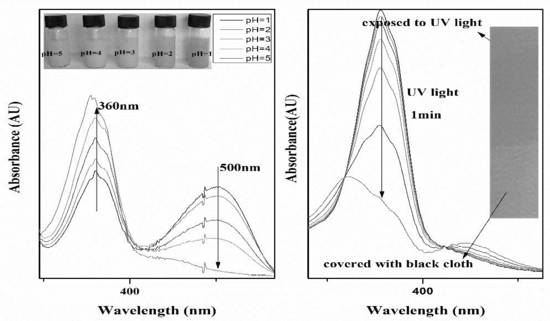
3.2.2. pH-Responsive Properties of the PMMAZO-Grafted CNCs
3.2.3. Light-Responsive Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Esteves, A.C.C.; Zhu, H.; Wang, D.; Xin, J.H. In-situ study of the structure and dynamics of thermo-responsive PNIPAAm grafted on a cotton fabric. Polymer 2012, 53, 3577–3586. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Habibi, Y.; Rojas, O.J.; Venditti, R.A.; Johansson, L.S.; Efimenko, K.; Osterberg, M.; Laine, J. Poly(N-isopropylacrylamide) brushes grafted from cellulose nanocrystals via surface-initiated single-electron transfer living radical polymerization. Biomacromolecules 2010, 11, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, H.; Hendrix, M.M.; Lousberg, N.J.; De With, G.; Esteves, A.C.; Xin, J.H. Temperature-triggered collection and release of water from fogs by a sponge-like cotton fabric. Adv. Mater. 2013, 25, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Kim, H.K.; Kim, T.; Bae, K.M.; Seo, S.M.; Kim, J.M.; Kang, T.J.; Yong, H.K. Self-Powered Humidity Sensor Based on Graphene Oxide Composite Film Intercalated by Poly(Sodium 4-Styrenesulfonate). ACS Appl. Mater. Interfaces 2014, 6, 8320–8326. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Usui, R.; Kitazawa, Y.; Lodge, T.P.; Watanabe, M. Thermally Reversible Ion Gels with Photohealing Properties Based on Triblock Copolymer Self-Assembly. Macromolecules 2015, 48, 5928–5933. [Google Scholar] [CrossRef]
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. pH-Responsive Cellulose Nanocrystal Gels and Nanocomposites. ACS Macro. Lett. 2012, 1, 1001–1006. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C Mater. 2013, 33, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 53001–53023. [Google Scholar] [CrossRef]
- Bashari, A.; Nejad, N.H.; Pourjavadi, A. Applications of stimuli responsive hydrogels: A textile engineering approach. J. Text. Inst. Proc. Abstr. 2013, 104, 1145–1155. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Baji, A.; Ramakrishna, S. Smart functional polymers—A new route towards creating a sustainable environment. Rsc Adv. 2014, 4, 53352–53364. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Schiphorst, J.T.; Broek, M.V.D.; Koning, T.D.; Murphy, J.N.; Schenning, A.P.H.J.; Esteves, A.C.C. Dual light and temperature responsive cotton fabric functionalized with a surface-grafted spiropyran–NIPAAm-hydrogel. J. Mater. Chem. A 2016, 4, 8676–8681. [Google Scholar] [CrossRef]
- Grigoray, O.; Wondraczek, H.; Heikkilä, E.; Fardim, P.; Heinze, T. Photoresponsive cellulose fibers by surface modification with multifunctional cellulose derivatives. Carbohyd. Polym. 2014, 111, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators. B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Yuan, T.; Dong, J.; Han, G.; Wang, G. Polymer nanoparticles self-assembled from photo-, pH- and thermo-responsive azobenzene-functionalized PDMAEMA. Rsc. Adv. 2016, 6, 10904–10911. [Google Scholar] [CrossRef]
- Yu, H.; Kobayashi, T. Photoresponsive block copolymers containing azobenzenes and other chromophores. Molecules 2010, 15, 570–603. [Google Scholar] [CrossRef] [PubMed]
- Berberova, N.; Daskalova, D.; Strijkova, V.; Kostadinova, D.; Nazarova, D.; Nedelchev, L.; Stoykova, E.; Marinova, V.; Chi, C.H.; Lin, S.H. Polarization holographic recording in thin films of pure azopolymer and azopolymer based hybrid materials. Opt. Mater. 2017, 64, 212–216. [Google Scholar] [CrossRef]
- Meng, X.; Natansohn, A.; Rochon, P. Azo polymers for reversible optical storage. 11 poly{4,4′-(1-methylethylidene)bisphenylene 3-[4-(4-nitrophenylazo)phenyl]-3-aza-pentanedioate}. J. Polym. Sci. Phys. 2015, 34, 1461–1466. [Google Scholar] [CrossRef]
- Xu, Q.; Yi, J.; Zhang, X.; Zhang, H. A novel amphotropic polymer based on cellulose nanocrystals grafted with azo polymers. Eur. Polym. J. 2008, 44, 2830–2837. [Google Scholar] [CrossRef]
- Sobolewska, A.; Bartkiewicz, S.; Miniewicz, A.; Schabbalcerzak, E. Polarization dependence of holographic grating recording in azobenzene-functionalized polymers monitored by visible and infrared light. J. Phys. Chem. B 2010, 114, 9751–9760. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Keller, P.; Li, B.; Wang, X.; Brunet, M. Light-Driven Side-On Nematic Elastomer Actuators. Adv. Mater. 2003, 15, 569–572. [Google Scholar] [CrossRef]
- Tang, X.; Gao, L.; Fan, X.; Zhou, Q. Controlled grafting of ethyl cellulose with azobenzene-containing polymethacrylates via atom transfer radical polymerization. J. Polym. Sci. Pol. Chem. 2010, 45, 1653–1660. [Google Scholar] [CrossRef]
- Duval, A.; Lange, H.; Lawoko, M.; Crestini, C. Modification of Kraft Lignin to Expose Diazobenzene Groups: Toward pH- and Light-Responsive Biobased Polymers. Biomacromolecules 2015, 16, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Meng, Q.; Liu, R.; Fu, S.; Lucia, L.A. Physical Study of the Primary and Secondary Photothermal Events in Gold/Cellulose Nanocrystals (AuNP/CNC) Nanocomposites Embedded in PVA Matrices. ACS Sustain. Chem. 2017, 5, 1601–1609. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Liu, N.; Guo, Z.; Singh, P.K.; Fu, S. Effect of surface wettability on the antibacterial activity of nanocellulose-based material with quaternary ammonium groups. Colloids Surf. A 2018, 554, 122–128. [Google Scholar] [CrossRef]
- Chen, J.; Yi, J.; Sun, P.; Liu, Z.T.; Liu, Z.W. Grafting from ramie fiber with poly(MMA) or poly(MA) via reversible addition-fragmentation chain transfer polymerization. Cellulose 2009, 16, 1133. [Google Scholar] [CrossRef]
- Xiao, M.; Li, S.; Chanklin, W.; Zheng, A.; Xiao, H. Surface-initiated atom transfer radical polymerization of butyl acrylate on cellulose microfibrils. Carbohyd. Polym. 2011, 83, 512–519. [Google Scholar] [CrossRef]
- Sui, X.; Yuan, J.; Zhou, M.; Zhang, J.; Yang, H.; Yuan, W.; Wei, Y.; Pan, C. Synthesis of cellulose-graft-poly(N,N-dimethylamino-2-ethyl methacrylate) copolymers via homogeneous ATRP and their aggregates in aqueous media. Biomacromolecules 2008, 9, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Bao, H.; Ou, K.; Yao, J.; Zhang, W.; He, J. Polymer-grafted modification of cotton fabrics by SI-ARGET ATRP. Fibers Polym. 2015, 16, 1478–1486. [Google Scholar] [CrossRef]
- Meng, T.; Gao, X.; Zhang, J.; Yuan, J.; Zhang, Y.; He, J. Graft copolymers prepared by atom transfer radical polymerization (ATRP) from cellulose. Polymer 2009, 50, 447–454. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Improving the reproducibility of chemical reactions on the surface of cellulose nanocrystals: ROP of ε-caprolactone as a case study. Cellulose 2011, 18, 607–617. [Google Scholar] [CrossRef]
- Liu, P.S.; Chen, Q.; Liu, X.; Yuan, B.; Wu, S.S.; Shen, J.; Lin, S.C. Grafting of zwitterion from cellulose membranes via ATRP for improving blood compatibility. Biomacromolecules 2009, 10, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wang, L. Preparation and characterization of antibacterial films based on polyvinyl alcohol/quaternized cellulose. React. Funct. Polym. 2016, 101, 90–98. [Google Scholar] [CrossRef]
- Cheng, B.; Ren, Y.; Wang, Y.; Ding, C. Synthesis and characterization of cellulose carbamate. J. Text. Res. 2007, 28, 1–4. [Google Scholar]
- Liu, X.; Chen, J.; Sun, P.; Liu, Z.W.; Liu, Z.T. Grafting modification of ramie fibers with poly(2,2,2-trifluoroethyl methacrylate) via reversible addition–fragmentation chain transfer (RAFT) polymerization in supercritical carbon dioxide. React. Funct. Polym. 2010, 70, 972–979. [Google Scholar] [CrossRef]
- Fernándezquiroz, D.; Gonzálezgómez, Á.; Lizardimendoza, J.; Vázquezlasa, B.; Goycoolea, F.M.; San, R.J.; Argüellesmonal, W.M. Effect of the molecular architecture on the thermosensitive properties of chitosan-g-poly(N-vinylcaprolactam). Carbohyd. Polym. 2015, 134, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kanaoka, S.; Aoshima, S. Photo-responsive copolymers with azobenzene side groups synthesized by living cationic polymerization: Efficient amplification of photosensitivity in aqueous photo-switching system. J. Polym. Sci. Pol. Chem. 2010, 43, 5337–5342. [Google Scholar] [CrossRef]
- Bandara, H.M.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]









| Sample | Conversion | Mn | PDI |
|---|---|---|---|
| PMMAZO-grafted CNCs | 32.5% | 18,791 | 1.27 |
| Sample | Degree of Crystallinity (%) |
|---|---|
| Pure CNCs | 76.21% |
| CNCs-IBBr | 66.49% |
| PMMAZO-grafted CNCs | 48.41% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, M.; Zheng, X.; Retulainen, E.; Fu, S. Dual Light- and pH-Responsive Composite of Polyazo-Derivative Grafted Cellulose Nanocrystals. Materials 2018, 11, 1725. https://doi.org/10.3390/ma11091725
Liu X, Li M, Zheng X, Retulainen E, Fu S. Dual Light- and pH-Responsive Composite of Polyazo-Derivative Grafted Cellulose Nanocrystals. Materials. 2018; 11(9):1725. https://doi.org/10.3390/ma11091725
Chicago/Turabian StyleLiu, Xiaohong, Ming Li, Xuemei Zheng, Elias Retulainen, and Shiyu Fu. 2018. "Dual Light- and pH-Responsive Composite of Polyazo-Derivative Grafted Cellulose Nanocrystals" Materials 11, no. 9: 1725. https://doi.org/10.3390/ma11091725