Comparative in Mechanical Behavior of 6061 Aluminum Alloy Welded by Pulsed GMAW with Different Filler Metals and Heat Treatments
Abstract
:1. Introduction
2. Materials and Methods
3. Results
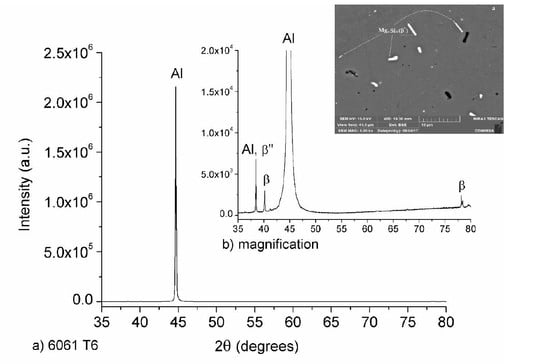
3.1. Microstructure and Phase Identification after Heat Treatment
3.2. Tensile Strenght Evaluation
3.3. Characterization of the Weld Joints
3.4. Fracture Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buchanan, K.; Colas, K.; Ribis, J.; Lopez, A.; Garnier, J. Analysis of the metastable precipitates in peak-hardness aged Al-Mg-Si(-Cu) alloys with differing Si contents. Acta Mater. 2017, 132, 209–221. [Google Scholar] [CrossRef]
- Farrell, K. Performance of Aluminum in Research Reactors. In Comprehensive Nuclear Materials; Elsevier: Karlsruhe, Germany, 2012; pp. 143–175. ISBN 978-0-08-056033-5. [Google Scholar]
- Fallah, V.; Korinek, A.; Raeisinia, B.; Gallerneault, M.; Esmaeili, S. Early-Stage Precipitation Phenomena and Composition-Dependent Hardening in Al-Mg-Si-(Cu) Alloys. Mater. Sci. Forum 2014, 794–796, 933–938. [Google Scholar] [CrossRef]
- Hirsch, J.; Al-Samman, T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications. Acta Mater. 2013, 61, 818–843. [Google Scholar] [CrossRef]
- Polmear, I.; St. John, D.; Ne, J.; Qian, M. Light Alloys: From Traditional Alloys to Nanocrystals; Elsevier: Oxford, UK, 2017; ISBN 978-0-08-099430-7. [Google Scholar]
- Prillhofer, R.; Rank, G.; Berneder, J.; Antrekowitsch, H.; Uggowitzer, P.; Pogatscher, S. Property Criteria for Automotive Al-Mg-Si Sheet Alloys. Materials 2014, 7, 5047–5068. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, F. Anwendungstechnologie Aluminium; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-662-43806-0. [Google Scholar]
- Chikhale, S.R.; Kolhe, K.P.; Kumar, P.; Kumar Verma, P. A Study of Microstructures of Gas Metal Arc Welded Aluminium Alloy 6061-T6. Int. J. Innov. Res. Sci. Technol. 2016, 3, 115–123. [Google Scholar]
- Patel, A.; Prasad, B.; Singh, D.K. Effect of variable process parameter of MIG welding on aluminium alloy 6061-T6. Int. J. Adv. Res. Ideas Innov. Technol. 2018, 4, 49–52. [Google Scholar]
- Missori, S.; Sili, A. Mechanical behaviour of 6082-T6 aluminum allow welds. Metall. Sci. Technol. 2000, 18, 3–7. [Google Scholar]
- Sasabe, S. Effect of Mn on welding liquation micro-cracking in heat affected zone of 6082 aluminum alloy. J. Jpn. Inst. Light Met. 2010, 60, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Torres, S.J. Evolución microestructural de la aleación de aluminio 6061 durante el proceso de soldadura MIG. Ing. Desarro. 2002, 52–65. [Google Scholar]
- Nie, F.; Dong, H.; Chen, S.; Li, P.; Wang, L.; Zhao, Z.; Li, X.; Zhang, H. Microstructure and Mechanical Properties of Pulse MIG Welded 6061/A356 Aluminum Alloy Dissimilar Butt Joints. J. Mater. Sci. Technol. 2018, 34, 551–560. [Google Scholar] [CrossRef]
- Myhr, O. Modelling of the age hardening behaviour of Al–Mg–Si alloys. Acta Mater. 2001, 49, 65–75. [Google Scholar] [CrossRef]
- Fallah, V.; Langelier, B.; Ofori-Opoku, N.; Raeisinia, B.; Provatas, N.; Esmaeili, S. Cluster evolution mechanisms during aging in Al–Mg–Si alloys. Acta Mater. 2016, 103, 290–300. [Google Scholar] [CrossRef]
- ASTM International ASTM B927/B917M—12 Standard Practice for Heat Treatment of Aluminum-Alloy Castings from All Processes. In ASTM Volume 02.02 Aluminum and Magnesium Alloys; ASTM International: West Conshohocken, PA, USA, 2012; Vol. 02.02.
- Atkins, G.; Thiessen, N.; Nissley, N.; Adonyi, Y. Welding Process Effects in Weldability Testing of Steels. Weld. J. 2002, 8, 61S–68S. [Google Scholar]
- Jang, K.C.; Lee, D.G.; Kuk, J.M.; Kim, I.S. Welding and environmental test condition effect in weldability and strength of Al alloy. J. Mater. Process. Technol. 2005, 164–165, 1038–1045. [Google Scholar] [CrossRef]
- BS EN 1011-1:1998—A2. Recommendations for Welding of Metallic Materials. Part 1: General Guidance for Arc Welding; A2 amendment.; Welding; European Committee for Standardization: London, UK, 2003.
- Weng, Y.; Jia, Z.; Ding, L.; Pan, Y.; Liu, Y.; Liu, Q. Effect of Ag and Cu additions on natural aging and precipitation hardening behavior in Al-Mg-Si alloys. J. Alloys Compd. 2017, 695, 2444–2452. [Google Scholar] [CrossRef]
- Guzmán, I.; Granda, E.; Vargas, B.; Cruz, C.; Avila, Y.; Acevedo, J. Tensile and fracture behavior in 6061-T6 and 6061-T4 aluminum alloys welded by pulsed metal transfer GMAW. Int. J. Adv. Manuf. Technol. 2019, 103, 2553–2562. [Google Scholar] [CrossRef]
- Chikhale, S.R.; Kolhe, K.P.; Kumar, P. Prediction of Mechanical properties of Al Alloy 6061-T6 by using GMAW. Int. J. Curr. Eng. Technol. 2016, 4, 300–306. [Google Scholar]
- ASTM International ASTM E3-11 (2017) Standard Guide for Preparation of Metallographic Specimens. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2017; Vol. 03.01.
- ASTM International ASTM E407-07 (2015)e1 Standard Practice for Microetching Metals and Alloys. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2015; Vol. 03.01.
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Styles, M.J.; Hutchinson, C.R.; Chen, Y.; Deschamps, A.; Bastow, T.J. The coexistence of two S (Al2CuMg) phases in Al–Cu–Mg alloys. Acta Mater. 2012, 60, 6940–6951. [Google Scholar] [CrossRef]
- ASTM E92-17 Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2017; Vol. 03.01.
- ASTM International ASTM E8/E8M—16a Standard Test Methods for Tension Testing of Metallic Materials. In ASTM Volume 03.01 Metals—Mechanical Testing; Elevated and Low-Temperature Tests; Metallography; ASTM International: West Conshohocken, PA, USA, 2016; Vol. 03.01.
- AWS D1.2/D1.2M Structural Welding Code—Aluminum; American Welding Society: Miami, FL, USA, 2013; pp. 83–87.
- Ding, L.; Jia, Z.; Nie, J.-F.; Weng, Y.; Cao, L.; Chen, H.; Wu, X.; Liu, Q. The structural and compositional evolution of precipitates in Al-Mg-Si-Cu alloy. Acta Mater. 2018, 145, 437–450. [Google Scholar] [CrossRef]
- Zandbergen, M.W.; Xu, Q.; Cerezo, A.; Smith, G.D.W. Study of precipitation in Al–Mg–Si alloys by Atom Probe Tomography I. Microstructural changes as a function of ageing temperature. Acta Mater. 2015, 101, 136–148. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Leitner, H.; Ebner, T.; Uggowitzer, P.J. Mechanisms controlling the artificial aging of Al–Mg–Si Alloys. Acta Mater. 2011, 59, 3352–3363. [Google Scholar] [CrossRef]
- Ehlers, F.J.H. Ab initio interface configuration determination for β″ in Al–Mg–Si: Beyond the constraint of a preserved precipitate stoichiometry. Comput. Mater. Sci. 2014, 81, 617–629. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Fan, C.; Cai, X.; Lin, S.; Yang, C. Analysis of droplet transfer, weld formation and microstructure in Al-Cu alloy bead welding joint with pulsed ultrasonic-GMAW method. J. Mater. Process. Technol. 2019, 271, 144–151. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Xu, C.; Yu, W.; Li, L. Precipitates Dissolution, Phase Transformation, and Re-precipitation-Induced Hardness Variation in 6082-T6 Alloy During MIG Welding and Subsequent Baking. JOM 2019, 71, 2711–2720. [Google Scholar] [CrossRef]
- Sevim, I.; Hayat, F.; Kaya, Y.; Kahraman, N.; Şahin, S. The study of MIG weldability of heat-treated aluminum alloys. Int. J. Adv. Manuf. Technol. 2013, 66, 1825–1834. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakaguchi, Y.; Miyata, Y.; Uetani, Y.; Sato, T.; Kamio, A.; Ikeno, S. Precipitation sequence of various kinds of metastable phases in Al-1.0mass% Mg2Si-0.4mass% Si alloy. J. Mater. Sci. 2000, 35, 179–189. [Google Scholar] [CrossRef]
- Saito, T.; Marioara, C.D.; Andersen, S.J.; Lefebvre, W.; Holmestad, R. Aberration-corrected HAADF-STEM investigations of precipitate structures in Al–Mg–Si alloys with low Cu additions. Philos. Mag. 2014, 94, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Hakem, M.; Lebaili, S.; Mathieu, S.; Miroud, D.; Lebaili, A.; Cheniti, B. Effect of microstructure and precipitation phenomena on the mechanical behavior of AA6061-T6 aluminum alloy weld. Int. J. Adv. Manuf. Technol. 2019, 102, 2907–2918. [Google Scholar] [CrossRef]
- Ambriz, R.R.; Barrera, G.; García, R.; López, V.H. A comparative study of the mechanical properties of 6061-T6 GMA welds obtained by the indirect electric arc (IEA) and the modified indirect electric arc (MIEA). Mater. Des. 2009, 30, 2446–2453. [Google Scholar] [CrossRef]
- Kaushal, C.; Sharma, L. To Determine Effects of Gas Metal Arc Welding (GMAW) Parameters on Mechanical Properties of Aluminium Alloys. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 4564–4572. [Google Scholar]
- Dawood, H.I.; Mohammed, K.S.; Rajab, M.Y. Advantages of the Green Solid State FSW over the Conventional GMAW Process. Adv. Mater. Sci. Eng. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]









| Alloy | Composition in wt % | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | |
| AA 6061 | 0.80 | 0.70 | 0.40 | 0.15 | 1.20 | 0.35 | 0.25 | 0.15 |
| ER 4043 | 6.00 | 0.80 | 0.30 | 0.05 | 0.05 | - | 0.10 | 0.20 |
| ER 5356 | 0.25 | 0.40 | 0.10 | 0.10 | 4.60 | 0.70 | 0.10 | 0.11 |
| Sample ID | HT | (V) | (A) | (mm/s) | (kJ/mm) | (kJ/mm) |
|---|---|---|---|---|---|---|
| M1-T4-5356 | T4 | 20 | 250 | 3.6171 | 1.3823 | 1.1888 |
| M2-T4-5356 | T4 | 20 | 270 | 2.9353 | 1.8397 | 1.5821 |
| M3-T4-5356 | T4 | 20 | 280 | 3.9357 | 1.4229 | 1.2237 |
| M4-T4-5356 | T4 | 20 | 295 | 3.7234 | 1.5846 | 1.3627 |
| M5-T6-5356 | T6 | 20 | 250 | 3.9978 | 1.2507 | 1.0756 |
| M6-T6-5356 | T6 | 20 | 270 | 3.8083 | 1.4180 | 1.2194 |
| M7-T6-5356 | T6 | 20 | 280 | 3.9347 | 1.4232 | 1.2240 |
| M8-T6-5356 | T6 | 20 | 295 | 3.8083 | 1.5493 | 1.3324 |
| M1-T4-4043 | T4 | 20 | 250 | 3.7310 | 1.3401 | 1.1525 |
| M2-T4-4043 | T4 | 20 | 270 | 3.8182 | 1.4143 | 1.2163 |
| M3-T4-4043 | T4 | 20 | 280 | 5.6000 | 1.0000 | 0.8600 |
| M4-T4-4043 | T4 | 20 | 295 | 3.9305 | 1.5011 | 1.2909 |
| M5-T6-4043 | T6 | 20 | 250 | 3.6567 | 1.3673 | 1.1759 |
| M6-T6-4043 | T6 | 20 | 270 | 4.0385 | 1.3371 | 1.1499 |
| M7-T6-4043 | T6 | 20 | 280 | 6.1000 | 0.9180 | 0.7895 |
| M8-T6-4043 | T6 | 20 | 295 | 3.8889 | 1.5171 | 1.3047 |
| Sample ID | UTS (MPa) | Rupture load (N) | Young’s Modulus (MPa) | Yield Stress (MPa) | Elongation (%) |
|---|---|---|---|---|---|
| M1-T4-5356 | 68.164 | 5449.32 | 44.260 | 44.2683 | 4.14 |
| M2-T4-5356 | 70.000 | 3192.86 | 48.327 | 26.4598 | 3.40 |
| M3-T4-5356 * | 110.151 | 8874.82 | 105.090 | 54.8868 | 6.49 |
| M4-T4-5356 * | 113.900 | 9175.48 | 94.134 | 56.7094 | 9.08 |
| M5-T6-5356 | 64.938 | 5183.30 | 93.378 | 27.9541 | 3.29 |
| M6-T6-5356 | 78.548 | 6429.39 | 82.937 | 33.5481 | 4.31 |
| M7-T6-5356 | 101.922 | 8219.11 | 94.904 | 46.5719 | 5.45 |
| M8-T6-5356 | 94.402 | 7612.49 | 82.248 | 70.1278 | 5.44 |
| M1-T4-4043 | 119.914 | 9503.50 | 94.775 | 70.5960 | 6.97 |
| M2-T4-4043 # | 153.932 | 12334.26 | 93.590 | 64.4380 | 16.6 |
| M3-T4-4043 | 104.543 | 7801.00 | 859.390 | 39.9000 | 39.00 |
| M4-T4-4043 | 135.708 | 10806.14 | 80.804 | 54.1100 | 12.53 |
| M5-T6-4043 | 130.140 | 10007.17 | 104.430 | 63.9230 | 3.79 |
| M6-T6-4043 | 71.665 | 5759.22 | 80.484 | 66.9950 | 2.23 |
| M7-T6-4043 | 64.000 | 4047.60 | 1315.650 | 35.8400 | 15.00 |
| M8-T6-4043 # | 153.583 | 12404.39 | 76.913 | 74.6320 | 23.82 |
| Sample ID | Weld Crown (mm) | Porosity Average Size (mm) | Weld Penetration (mm) |
|---|---|---|---|
| M1-T4-5356 | 1.6768 | 0.044125 | 8.3436 |
| M2-T4-5356 | 3.0540 | 0.048770 | 10.3155 |
| M3-T4-5356 | 1.2520 | 0.035675 | 8.5314 |
| M4-T4-5356 | 1.7662 | 0.046635 | 8.3883 |
| M5-T6-5356 | 0.5008 | 0.049290 | 11.250 |
| M6-T6-5356 | 1.5739 | 0.045095 | 8.9115 |
| M7-T6-5356 | 1.7886 | 0.067285 | 8.6475 |
| M8-T6-5356 | 1.2207 | 0.033605 | 8.9115 |
| M1-T4-4043 | 0.2638 | 0.045755 | 9.2268 |
| M2-T4-4043 | 0.8658 | 0.043840 | 8.2528 |
| M3-T4-4043 | 0.7674 | 0.007700 | 8.1362 |
| M4-T4-4043 | 0.8500 | 0.060000 | 8.2536 |
| M5-T6-4043 | 0 | 0.069310 | 9.1058 |
| M6-T6-4043 | 0 | 0.049385 | 6.6030 |
| M7-T6-4043 | 0.7087 | 0.010900 | 5.9249 |
| M8-T6-4043 | 1.5516 | 0.046085 | 8.3436 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, I.; Granda, E.; Acevedo, J.; Martínez, A.; Dávila, Y.; Velázquez, R. Comparative in Mechanical Behavior of 6061 Aluminum Alloy Welded by Pulsed GMAW with Different Filler Metals and Heat Treatments. Materials 2019, 12, 4157. https://doi.org/10.3390/ma12244157
Guzmán I, Granda E, Acevedo J, Martínez A, Dávila Y, Velázquez R. Comparative in Mechanical Behavior of 6061 Aluminum Alloy Welded by Pulsed GMAW with Different Filler Metals and Heat Treatments. Materials. 2019; 12(24):4157. https://doi.org/10.3390/ma12244157
Chicago/Turabian StyleGuzmán, Isidro, Everardo Granda, Jorge Acevedo, Antonia Martínez, Yuliana Dávila, and Rubén Velázquez. 2019. "Comparative in Mechanical Behavior of 6061 Aluminum Alloy Welded by Pulsed GMAW with Different Filler Metals and Heat Treatments" Materials 12, no. 24: 4157. https://doi.org/10.3390/ma12244157