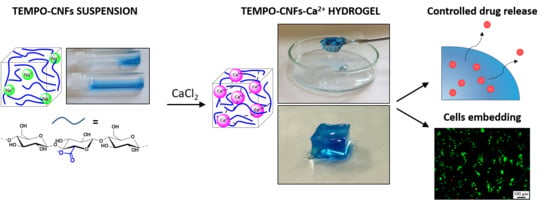
TEMPO-Nanocellulose/Ca2+ Hydrogels: Ibuprofen Drug Diffusion and In Vitro Cytocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogels Preparation and Characterization
2.1.1. Synthesis
2.1.2. Rheology
2.1.3. High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance (HR-MAS NMR) Spectroscopy
2.1.4. In Vitro Characterization
3. Results and Discussion
3.1. Rheological Properties
3.2. Ibuprofen Drug Diffusion in Hydrogels
3.3. Cytocompatibility of 2,2,6,6-Tetramethylpiperidine 1-oxyl (TEMPO)-Oxidized and Ultra-Sonicated Cellulose Nanofibers (TOUS-CNFs)/Ca2+ Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Gilman, A.G.; Goodman, L.S.; Macmillan, a.g. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 6th ed.; Blackwell Scientific Publications: New York, NY, USA, 1980; pp. 18–26. [Google Scholar]
- Mauri, E.; Rossetti, A.; Mozetic, P.; Schiavon, C.; Sacchetti, A.; Rainer, A.; Rossi, F. Ester coupling of ibuprofen in hydrogel matrix: A facile one-step strategy for controlled anti-inflammatory drug release. Eur. J. Pharm. Biopharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Naso, D.; Rossetti, A.; Borghi, E.; Ottaviano, E.; Griffini, G.; Masi, M.; Sacchetti, A.; Rossi, F. Design of polymer-based antimicrobial hydrogels through physico-chemical transition. Mater. Sci. Eng. C 2019, 103, 109791. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Sau, C.; Lai, W.; Cichon, J.; Li, W. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1–34. [Google Scholar]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Paul Solomon, F.D. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Ceriani, F.; Draghi, L. 3D Encapsulation Made Easy: A Coaxial-Flow Circuit for the Fabrication of Hydrogel Microfibers Patches. Bioengineering 2019, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Contessi Negrini, N.; Bonetti, L.; Contili, L.; Farè, S. 3D printing of methylcellulose-based hydrogels. Bioprinting 2018, 10, e00024. [Google Scholar] [CrossRef]
- Torres, A.L.; Bidarra, S.J.; Pinto, M.T.; Aguiar, P.C.; Silva, E.A.; Barrias, C.C. Guiding morphogenesis in cell-instructive microgels for therapeutic angiogenesis. Biomaterials 2018, 154, 34–47. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Sakai, S.; Ashida, T.; Taya, M. Production of hyaluronic-acid-based cell-enclosing microparticles and microcapsules via enzymatic reaction using a microfluidic system. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Caron, I.; Rossi, F.; Papa, S.; Aloe, R.; Sculco, M.; Mauri, E.; Sacchetti, A.; Erba, E.; Panini, N.; Parazzi, V.; et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials 2016, 75, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Vismara, I.; Mariani, A.; Barilani, M.; Rimondo, S.; De Paola, M.; Panini, N.; Erba, E.; Mauri, E.; Rossi, F.; et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J. Control. Release 2018, 278, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Choudhury, N.R. 3D bioprinted nanocellulose-based hydrogels for tissue engineering applications: A brief review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized cellulose—Survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S. Cellulose: To depolymerize… or not to? Biotechnol. Adv. 2017, 35, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Fiorati, A.; Pastori, N.; Punta, C.; Melone, L. Sponge-like functional materials from TEMPO-oxidized cellulose nanofibers. In Nanosponges: From Fundamentals to Applications; Mele, A., Trotta, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; ISBN 978-3-527-34099-6. [Google Scholar]
- Fiorati, A.; Grassi, G.; Graziano, A.; Liberatori, G.; Pastori, N.; Melone, L.; Bonciani, L.; Pontorno, L.; Punta, C.; Corsi, I. Eco-design of nanostructured cellulose sponges for sea-water decontamination from heavy metal ions. J. Clean. Prod. 2020, 246, 119009. [Google Scholar] [CrossRef]
- Melone, L.; Altomare, L.; Alfieri, I.; Lorenzi, A.; De Nardo, L.; Punta, C. Ceramic aerogels from TEMPO-oxidized cellulose nanofibre templates: Synthesis, characterization, and photocatalytic properties. J. Photochem. Photobiol. A Chem. 2013, 261, 53–60. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Sganappa, A.; Melone, L.; Punta, C.; Cametti, M. Naked-Eye Heterogeneous Sensing of Fluoride Ions by Co-Polymeric Nanosponge Systems Comprising Aromatic-Imide-Functionalized Nanocellulose and Branched Polyethyleneimine. ChemPlusChem 2019, 84, 1512–1518. [Google Scholar] [CrossRef]
- Fiorati, A.; Turco, G.; Travan, A.; Caneva, E.; Pastori, N.; Cametti, M.; Punta, C.; Melone, L. Mechanical and drug release properties of sponges from cross-linked cellulose nanofibers. ChemPlusChem 2017, 82, 848–858. [Google Scholar] [CrossRef]
- Panzella, L.; Melone, L.; Pezzella, A.; Rossi, B.; Pastori, N.; Perfetti, M.; D’Errico, G.; Punta, C.; d’Ischia, M. Surface-Functionalization of Nanostructured Cellulose Aerogels by Solid State Eumelanin Coating. Biomacromolecules 2016, 17, 564–571. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chemie Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Paladini, G.; Venuti, V.; Almásy, L.; Melone, L.; Crupi, V.; Majolino, D.; Pastori, N.; Fiorati, A.; Punta, C. Cross-linked cellulose nano-sponges: A small angle neutron scattering (SANS) study. Cellulose 2019, 26, 9005–9019. [Google Scholar] [CrossRef]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. J. Colloid Interface Sci. 2018, 509, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Snyder, J.F.; Williams, K.S.; Andzelm, J.W. Cation-Induced Hydrogels of Cellulose Nanofibrils with Tunable Moduli. Biomacromolecules 2013, 14, 3338–3345. [Google Scholar] [CrossRef] [PubMed]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal Cation Cross-Linked Nanocellulose Hydrogels as Tissue Engineering Substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Peng, X.; Zhan, C.; Naderi, A.; Sharma, P.R.; Mao, Y.; Hsiao, B.S. Structure characterization of cellulose nanofiber hydrogel as functions of concentration and ionic strength. Cellulose 2017, 24, 5417–5429. [Google Scholar] [CrossRef]
- Masruchin, N.; Park, B.-D.; Causin, V.; Um, I.C. Characteristics of TEMPO-oxidized cellulose fibril-based hydrogels induced by cationic ions and their properties. Cellulose 2015, 22, 1993–2010. [Google Scholar] [CrossRef]
- Torres-Rendon, J.G.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M.; et al. Bioactive Gyroid Scaffolds Formed by Sacrificial Templating of Nanocellulose and Nanochitin Hydrogels as Instructive Platforms for Biomimetic Tissue Engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef]
- Hua, K.; Rocha, I.; Zhang, P.; Gustafsson, S.; Ning, Y.; Strømme, M.; Mihranyan, A.; Ferraz, N. Transition from Bioinert to Bioactive Material by Tailoring the Biological Cell Response to Carboxylated Nanocellulose. Biomacromolecules 2016, 17, 1224–1233. [Google Scholar] [CrossRef]
- Rashad, A.; Mustafa, K.; Heggset, E.B.; Syverud, K. Cytocompatibility of Wood-Derived Cellulose Nanofibril Hydrogels with Different Surface Chemistry. Biomacromolecules 2017, 18, 1238–1248. [Google Scholar] [CrossRef]
- Martínez Ávila, H.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1–2, 22–35. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Jiang, Y.; Zhang, Q.; Shi, H.; Liu, D. 3D printing process of oxidized nanocellulose and gelatin scaffold. J. Biomater. Sci. Polym. Ed. 2018, 29, 1498–1513. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Lungu, A.; Stancu, I.C.; Serafim, A.; Heggset, E.; Syverud, K.; Iovu, H. Bioinspired 3D printable pectin-nanocellulose ink formulations. Carbohydr. Polym. 2019, 220, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Casalegno, M.; Ferro, M.; Rossi, F.; Raos, G.; Mele, A. Evidence of superdiffusive nanoscale motion in anionic polymeric hydrogels: Analysis of PGSE- NMR data and comparison with drug release properties. J. Control. Release 2019, 305, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zubkov, M.; Dennis, G.R.; Stait-Gardner, T.; Torres, A.M.; Willis, S.A.; Zheng, G.; Price, W.S. Physical characterization using diffusion NMR spectroscopy. Magn. Reson. Chem. 2017, 55, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Marchetti, P.; Rossi, F.; Perale, G.; Castiglione, F.; Mele, A.; Masi, M. Smart approach to evaluate drug diffusivity in injectable agar-carbomer hydrogels for drug delivery. J. Phys. Chem. B 2011, 115, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.M.; Hibbs, M.R. Characterization of heterogeneous solvent diffusion environments in anion exchange membranes. Macromolecules 2014, 47, 1073–1084. [Google Scholar] [CrossRef]
- Violette, A.; Lancelot, N.; Poschalko, A.; Piotto, M.; Briand, J.P.; Raya, J.; Elbayed, K.; Bianco, A.; Guichard, G. Exploring helical folding of oligoureas during chain elongation by high-resolution magic-angle-spinning (HRMAS) NMR spectroscopy. Chem. A Eur. J. 2008, 14, 3874–3882. [Google Scholar] [CrossRef]
- Beckonert, O.; Coen, M.; Keun, H.C.; Wang, Y.; Ebbels, T.M.D.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat. Protoc. 2010, 5, 1019–1032. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; Guguen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef]
- ISO 10993-5:2009, Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 1 January 2020).
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Brandl, F.; Kastner, F.; Gschwind, R.M.; Blunk, T.; Teßmar, J.; Göpferich, A. Hydrogel-based drug delivery systems: Comparison of drug diffusivity and release kinetics. J. Control. Release 2010, 142, 221–228. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Punta, C.; Melone, L.; Panzeri, W.; Rossi, B.; Trotta, F.; Mele, A. Anomalous diffusion of ibuprofen in cyclodextrin nanosponge hydrogels: An HRMAS NMR study. Beilstein J. Org. Chem. 2014, 10, 2715–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainsford, K.D. Ibuprofen: Discovery, Development and Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Manoukian, M.A.C.; Migdal, C.W.; Tembhekar, A.R.; Harris, J.A.; DeMesa, C. Topical Administration of Ibuprofen for Injured Athletes: Considerations, Formulations, and Comparison to Oral Delivery. Sports Med. Open 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebi, D.; Guy, R.H.; Edler, K.J.; Scott, J.L. Ibuprofen delivery into and through the skin from novel oxidized cellulose-based gels and conventional topical formulations. Int. J. Pharm. 2016, 514, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewster, M.E.; Loftsson, T. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar]
- Cohen, Y.; Avram, L.; Frish, L. Diffusion NMR spectroscopy in supramolecular and combinatorial chemistry: An old parameter—New insights. Angew. Chemie Int. Ed. 2005, 44, 520–554. [Google Scholar] [CrossRef]
- Bellia, F.; La Mendola, D.; Pedone, C.; Rizzarelli, E.; Saviano, M.; Vecchio, G. Selectively functionalized cyclodextrins and their metal complexes. Chem. Soc. Rev. 2009, 38, 2756–2781. [Google Scholar] [CrossRef]
- Harper, B.J.; Clendaniel, A.; Sinche, F.; Way, D.; Hughes, M.; Schardt, J.; Simonsen, J.; Stefaniak, A.B.; Harper, S.L. Impacts of chemical modification on the toxicity of diverse nanocellulose materials to developing zebrafish. Cellulose 2016, 23, 1763–1775. [Google Scholar] [CrossRef] [Green Version]
- Ventura, C.; Lourenço, A.F.; Sousa-Uva, A.; Ferreira, P.J.T.; Silva, M.J. Evaluating the genotoxicity of cellulose nanofibrils in a co-culture of human lung epithelial cells and monocyte-derived macrophages. Toxicol. Lett. 2018, 291, 173–183. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
- de Carvalho, R.A.; Veronese, G.; Carvalho, A.J.F.; Barbu, E.; Amaral, A.C.; Trovatti, E. The potential of TEMPO-oxidized nanofibrillar cellulose beads for cell delivery applications. Cellulose 2016, 23, 3399–3405. [Google Scholar] [CrossRef] [Green Version]
- Bidarra, S.J.; Oliveira, P.; Rocha, S.; Saraiva, D.P.; Oliveira, C.; Barrias, C.C. A 3D in vitro model to explore the inter-conversion between epithelial and mesenchymal states during EMT and its reversion. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.F.; Mariano, M.; Reis, D.; Lombello, C.B.; Ferreira, M.; Sain, M. Cell interactions and cytotoxic studies of cellulose nanofibers from Curauá natural fibers. Carbohydr. Polym. 2018, 201, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| ID | TOUS-CNFs Concentration (w/v) (%) | Cellulose (g) | NaOH 0.1 M (mL) | Water (mL) |
|---|---|---|---|---|
| A | 0.5 | 0.2 | 3.02 | 36.98 |
| B | 1 | 0.4 | 6.04 | 33.96 |
| C | 2 | 0.8 | 12.08 | 27.92 |
| D | 4 | 1.6 | 24.16 | 15.84 |
| ID | Cellulose Concentration (w/v) (%) | CaCl2 Final Concentration (mM) | TOUS-CNFs Dispersion (mg) | CaCl2 Solution 100 mM (µL) | Water (µL) |
|---|---|---|---|---|---|
| A1 | 0.5 | 10 | 450 | 50 | 0 |
| A2 | 0.5 | 5 | 450 | 25 | 25 |
| A3 | 0.5 | 2 | 450 | 10 | 40 |
| A4 | 0.5 | 0 | 450 | 0 | 50 |
| B1 | 1 | 10 | 450 | 50 | 0 |
| B2 | 1 | 5 | 450 | 25 | 25 |
| B3 | 1 | 2 | 450 | 10 | 40 |
| B4 | 1 | 0 | 450 | 0 | 50 |
| C1 | 2 | 10 | 450 | 50 | 0 |
| C2 | 2 | 5 | 450 | 25 | 25 |
| C3 | 2 | 2 | 450 | 10 | 40 |
| C4 | 2 | 0 | 450 | 0 | 50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorati, A.; Contessi Negrini, N.; Baschenis, E.; Altomare, L.; Faré, S.; Giacometti Schieroni, A.; Piovani, D.; Mendichi, R.; Ferro, M.; Castiglione, F.; et al. TEMPO-Nanocellulose/Ca2+ Hydrogels: Ibuprofen Drug Diffusion and In Vitro Cytocompatibility. Materials 2020, 13, 183. https://doi.org/10.3390/ma13010183
Fiorati A, Contessi Negrini N, Baschenis E, Altomare L, Faré S, Giacometti Schieroni A, Piovani D, Mendichi R, Ferro M, Castiglione F, et al. TEMPO-Nanocellulose/Ca2+ Hydrogels: Ibuprofen Drug Diffusion and In Vitro Cytocompatibility. Materials. 2020; 13(1):183. https://doi.org/10.3390/ma13010183
Chicago/Turabian StyleFiorati, Andrea, Nicola Contessi Negrini, Elena Baschenis, Lina Altomare, Silvia Faré, Alberto Giacometti Schieroni, Daniele Piovani, Raniero Mendichi, Monica Ferro, Franca Castiglione, and et al. 2020. "TEMPO-Nanocellulose/Ca2+ Hydrogels: Ibuprofen Drug Diffusion and In Vitro Cytocompatibility" Materials 13, no. 1: 183. https://doi.org/10.3390/ma13010183