Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review
Abstract
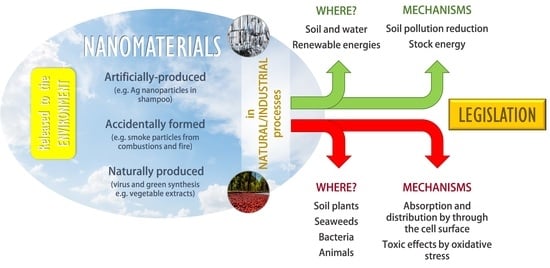
:1. Introduction
- Accidentally formed nanomaterials: They appear as a subsequent product from industrial or natural processes, such as combustions (e.g., smokes from cigarettes or fires).
- Artificially produced nanomaterials: They are designed by humans with determined properties and characteristics (e.g., Ag NPs in shampoos) [9]. The main difference between accidentally formed nanomaterials is that these ones are intended to be formed with chosen sizes and composition, like their characteristics, while accidentally formed nanomaterials appear in a natural and spontaneous way.
- Naturally produced nanomaterials: They can be found in living beings and nature (e.g., viruses). The line that separates natural or accidental materials is, in certain occasions, extremely difficult to distinguish [10].
- Antibacterial activity: The threatening increase of microorganisms resistance towards antibiotics has become an important concern, where metallic NPs can make the difference as an alternative or adjuvant treatment due to their antibacterial properties [11]. For example, zinc oxide inhibits and prevents Staphylococcus aureus growth. Unfortunately, this mechanism is still not fully understood [12], although ZnNPs has been described to cause growth inhibition in bacteria via reactive oxygen species (ROS) production, specifically H2O2 [13]. In E. coli, these nanoparticles are accumulated in the membrane [14], generating electrostatic charges that cause critical damage [15].
- Drug delivery systems: Delivering a drug to a specific site where it is meant to exert its effect is one of the greatest nanotechnology promises [16], becoming especially important when it is used for cancer treatment in order to avoid associated side effects and problems. Interestingly, there are some approved therapies based on the use of these technologies, such as albumin NPs [17], for example, Abraxane® [18]. In particular, the enhanced permeability and retention effect (EPR effect) plays an important role in the biological distribution of NPs, granting high drug concentration in tumor cells against very low drug concentration in healthy tissues, which results in a higher therapeutic effect and desirable toxicity [19]. The possibility of transporting different substances at once granted by nanomaterials is a stimulating advantage and a potential solution for multiple therapies [20].
- Food preservation: Some NPs’ properties allow them to form an impassable barrier against gases, humidity and other factors that could alter and reduce food stability. Furthermore, the food decomposition preventing effect could be complemented due to the antibacterial and antioxidant activities that some NPs possess [21].
- Sunscreen: Titanium dioxide (TiO2) and zinc dioxide (ZnO) in a nanometric scale are extremely effective at absorbing ultraviolet light [22], which makes them a valuable component for sunscreens and solar protection products.
- Molecular detection and diagnosis: The use of magnetic NPs for detecting specific molecules inside the organism has accomplished a lot of achievements, making it possible to identify whether pathogens are invading the organism [23] or molecules related to inborn genetic defects [24,25]. For instance, gold NPs (Au NPs) are used in different biological analysis processes, such as diagnosing patients with possible allergy problems (InmunoCAP®, ImmunoCap™ Phadiatop®, Phadia AB, Upssala, Sweden) or as highly visible indicators in pregnancy tests (First Response®, Church and Dwight Co. Inc., Princeton, NJ, USA) due to their optical properties and their chemical stability [26]. Gadolinium NPs (Gd NPs) are also widely used in cancer diagnosis [27].
- Sports equipment: carbon nanotubes allow enhancing equipment and tools by improving resistance and flexibility, granting and enduring a more effective product [28].
- Water purification: iron NPs slightly enriched with Palladium have the capacity to eliminate organic chlorine in waters and soils [30].
2. Nanoparticles Emission
2.1. Product Fabrication
2.2. Product Employment
2.3. Disposal and Recycling
- Incinerated products: Their incineration causes the appearance of ash particles in the air, and NPs could form an important part of their composition [41]. However, the efficiency of the filters present in incineration plants (higher than 99.6%) causes an extremely small direct emission of these particles to the air [51].
2.4. Transformation
- A.
- Photochemical transformation: depending on the incident wavelength, the penetration capacity on the product and the nanomaterial photosensitivity, the excitation produced in the NPs and their consequent transformation can be greater or lower. For example, the interaction of TiO2 NPs with sunlight produces the appearance of ROS in living organisms, causing an increase in toxicity [37].
- B.
- Oxidation and reduction: these processes can occur when the reaction is thermodynamically favored, so they depend on the medium and conditions surrounding the nanomaterial (pH, presence of oxidizing and/or reducing agents, reagents, or stabilizers). For example, the oxidation of Ag0 to Ag+ is a consequence of washing clothes and fabrics that include NPs made of this material. This fact increases their toxicity, in addition to reducing their effectiveness in the product [56].
- C.
- Dissolution and precipitation: the dissolution of ions or water-soluble molecules can occur, but also subsequent precipitation of a new solid which contains, in addition to NPs, other ligands naturally present in the water. Therefore, dissolution and precipitation processes are transformations that may happen independently or consecutively. For example, the dissolution of metallic NPs made of Cu or Zn increases their bioavailability and, therefore, their toxicity [57].
- D.
- Adsorption and desorption: adsorption to solids can take place through Van der Waals forces, electrostatic interactions, or chemical bonds, while changes in the balance between products cause desorption. For example, graphene oxide (GO) NPs may adsorb antibiotics such as levofloxacin, manipulating their mobility, transport and effect, increasing their risk of toxicity [37].
- E.
- Combustion: high temperatures processes, such as incineration, lead to combustion reactions that can chemically modify the NPs. For example, iron NPs in spontaneous coal combustion affects climatic variables [58].
- F.
- Biotransformation: it includes all the processes listed above (except combustion) when they are mediated by biological agents. For example, myeloperoxidase in humans degrades graphene oxide NPs, reducing their cytotoxicity [37].
- G.
- Abrasion: Common handling of some products can produce the release of NPs contained in their structure. For example, building materials coatings or polyurethane coatings can release NPs after long-term employment [55].
2.5. Evaluating Nanomaterial Release
3. Environmental Negative Impact
3.1. Absorption and Distribution
3.2. Mechanisms of Toxicity: Oxidative Stress
3.3. Toxicity in Bacteria Studies
3.4. Toxicity in Soil Plants and Seaweeds Studies
3.5. Toxicity Animal Studies
3.6. Green Nanotechnology Studies
3.7. Vegetable Extracts
4. Environmental Positive Impact
4.1. Soil and Water Contamination
4.2. Energetical Applications
4.3. Other Applications
5. Legislation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Accorsi, G.; Verri, G.; Bolognesi, M.; Armaroli, N.; Clementi, C.; Miliani, C.; Romani, A. The exceptional near-infrared luminescence properties of cuprorivaite (Egyptian blue). Chem. Commun. 2009, 3392–3394. [Google Scholar] [CrossRef]
- Link, S.; Zhong, L.; Wang, A.Z.L.; Mostafa, A.E.-S. How Does a Gold Nanorod Melt? J. Phys. Chem. B 2000, 104, 7867–7870. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Benelmekki, M. Designing Hybrid Nanoparticles, 1st ed.; Morgan & Claypool: Bristol, UK, 2015. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strambeanu, N.; Demetrovici, L.; Dragos, D.; Lungu, M. Nanoparticles: Definition, Classification and General Physical Properties. In Nanoparticles’ Promises and Risks: Characterization, Manipulation, and Potential Hazards to Humanity and the Environment; Lungu, M., Neculae, A., Bunoiu, M., Biris, C., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–8. [Google Scholar] [CrossRef]
- Tweney, R.D. Discovering Discovery: How Faraday Found the First Metallic Colloid. Perspect. Sci. 2006, 14, 97–121. [Google Scholar] [CrossRef]
- Ochekpe, N.A.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and Drug Delivery Part 1: Background and Applications. Trop. J. Pharm. Res. 2009, 8, 265–274. [Google Scholar] [CrossRef]
- Miranzadeh, M.; Kassaee, M.; Afshari, F. Efficiency of Cu, Ag, and Fe Nanoparticles As Detergents Preservatives Against E. coli and S. aureus. Nanochem. Res. 2019, 4, 170–178. [Google Scholar] [CrossRef]
- Cao, S.; Rathi, P.; Wu, X.; Ghim, D.; Jun, Y.; Singamaneni, S. Cellulose Nanomaterials in Interfacial Evaporators for Desalination: A “Natural” Choice. Adv. Mater. 2020. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Hu, C.; Shao, L.Q. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Sun, T.M.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.X.; Xia, Y.N. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew Chemie-Internationa. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Lu, P.J.; Fang, S.W.; Cheng, W.L.; Huang, S.C.; Huang, M.C.; Cheng, H.F. Characterization of titanium dioxide and zinc oxide nanoparticles in sunscreen powder by comparing different measurement methods. J. Food Drug Anal. 2018, 26, 1192–1200. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutierrez, L.; Morales, M.P.; Bohm, I.B. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of Nanoparticles in Biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.; García-Martín, M.L.; Pernia Leal, M. Manganese-Based Nanogels as pH Switches for Magnetic Resonance Imaging. Biomacromolecules 2017, 18, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, S.C.; Kurland, B.F.; Liu, C.-L.; Ho, R.J.Y.; Ruddell, A. Tumor-induced lymph node alterations detected by MRI lymphography using gadolinium nanoparticles. Sci. Rep. 2015, 5, 15641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaine, S.J.; Koelmans, A.A.; Horne, N.; Carley, S.; Handy, R.D.; Kapustka, L.; Nowack, B.; von der Kammer, F. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mahian, O.; Kianifar, A.; Kalogirou, S.A.; Pop, I.; Wongwises, S. A review of the applications of nanofluids in solar energy. Int. J. Heat Mass Transf. 2013, 57. [Google Scholar] [CrossRef]
- Aiello, A.; Morey, J.R.; Livi, K.J.T.; DeLong, H.C.; ElBidweihy, H.; Trulove, P.C.D.; David, P. Lignocellulose-stabilized iron-palladium nanomagnetic biocomposites. J. Magnet. Magnet. Mater. 2020, 497, 165964. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Grillo, R.; Fraceto, L.F.; Amorim, M.J.B.; Scott-Fordsmand, J.J.; Schoonjans, R.; Chaudhry, Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J. Hazard. Mater. 2021, 404, 124148. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110347. [Google Scholar] [CrossRef] [PubMed]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Kaviyani, F.E.; Naeemi, A.K.; Salehzadeh, A. Acute toxicity and effects of titanium dioxide nanoparticles (TiO2 NPs) on some metabolic enzymes and hematological indices of the endangered Caspian trout juveniles (Salmo trutta caspius Kessler, 1877). Iran. J. Fish. Sci. 2020, 19, 1253–1267. [Google Scholar] [CrossRef]
- Correia, A.; Briones, F. NanoSpain. Available online: http://www.nanospain.org/nanospain.php?p=h (accessed on 12 December 2020).
- Abbas, Q.; Yousaf, B.; Ali, M.U.; Munir, M.A.M.; El-Naggar, A.; Rinklebe, J.; Naushad, M. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Environ. Int. 2020, 138, 105646. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Dong, H.; Li, L.; Wang, Y.; Ning, Q.; Wang, B.; Zeng, G. Aging of zero-valent iron-based nanoparticles in aqueous environment and the consequent effects on their reactivity and toxicity. Water Environ. Res. 2020, 92, 646–661. [Google Scholar] [CrossRef]
- Rajkovic, S.; Bornhöft, N.A.; van der Weijden, R.; Nowack, B.; Adam, V. Dynamic probabilistic material flow analysis of engineered nanomaterials in European waste treatment systems. Waste Manag. 2020, 113, 118–131. [Google Scholar] [CrossRef]
- Nowack, B. Evaluation of environmental exposure models for engineered nanomaterials in a regulatory context. NanoImpact 2017, 8, 38–47. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef]
- Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, S.N.; Wigger, H.; Zabeo, A.; Semenzin, E.; Hristozov, D.; Nowack, B.; Spurgeon, D.J.; Baun, A. Comparison of species sensitivity distribution modeling approaches for environmental risk assessment of nanomaterials—A case study for silver and titanium dioxide representative materials. Aquatic Toxicol. 2020, 225. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Medhi, S.; Chowdhury, S.; Gupta, D.K.; Mazumdar, A. An investigation on the effects of silica and copper oxide nanoparticles on rheological and fluid loss property of drilling fluids. J. Petroleum Exploration Prod. Technol. 2020, 10, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Veksha, A.; Latiff, N.M.; Chen, W.; Ng, J.E.; Lisak, G. Heteroatom doped carbon nanosheets from waste tires as electrode materials for electrocatalytic oxygen reduction reaction: Effect of synthesis techniques on properties and activity. Carbon 2020, 167, 104–113. [Google Scholar] [CrossRef]
- Tada, S.; Fujiwara, K.; Yamamura, T.; Nishijima, M.; Uchida, S.; Kikuchi, R. Flame spray pyrolysis makes highly loaded Cu nanoparticles on ZrO2 for CO2-to-methanol hydrogenation. Chem. Eng. J. 2020, 381, 122750. [Google Scholar] [CrossRef]
- Nowack, B.; David, R.M.; Fissan, H.; Morris, H.; Shatkin, J.A.; Stintz, M.; Zepp, R.; Brouwer, D. Potential release scenarios for carbon nanotubes used in composites. Environ. Int. 2013, 59, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, A.J.; Jensen, A.C.Ø.; Kling, K.I.; Nørgaard, A.; Brinch, A.; Christensen, F.; Jensen, K.A. Quantitative material releases from products and articles containing manufactured nanomaterials: Towards a release library. NanoImpact 2017, 5, 119–132. [Google Scholar] [CrossRef]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [Green Version]
- Thurber, A.P.; Alanko, G.; Beausoleil II, G.L.; Dodge, K.N.; Hanna, C.B.; Punnoose, A. Unusual crystallite growth and modification of ferromagnetism due to aging in pure and doped ZnO nanoparticles. J. Appl. Phys. 2012, 111, 07C319. [Google Scholar] [CrossRef] [Green Version]
- Nowack, B.; Ranville, J.F.; Diamond, S.; Gallego-Urrea, J.A.; Metcalfe, C.; Rose, J.; Horne, N.; Koelmans, A.A.; Klaine, S.J. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.B.; Zaikova, T.; Barber, A.; Simonich, M.; Lankone, R.; Marco, M.; Hristovski, K.; Herckes, P.; Passantino, L.; Fairbrother, D.H.; et al. Potential Environmental Impacts and Antimicrobial Efficacy of Silver- and Nanosilver-Containing Textiles. Environ Sci. Technol. 2016, 50, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total. Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Pinto, D.; Lima, B.D. Implications of iron nanoparticles in spontaneous coal combustion and the effects on climatic variables. Chemosphere 2020, 254, 126814. [Google Scholar] [CrossRef]
- Dale, A.L.; Casman, E.A.; Lowry, G.V.; Lead, J.R.; Viparelli, E.; Baalousha, M. Modeling nanomaterial environmental fate in aquatic systems. Environ. Sci. Technol. 2015, 49, 2587–2593. [Google Scholar] [CrossRef]
- Ma, X.; Geisler-Lee, J.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total. Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.M.; Webb, T.R. Comparative Pulmonary Toxicity Assessment of Single-wall Carbon Nanotubes in Rats. Toxicol. Sci. 2004, 77, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.J.; Shaw, B.J.; Handy, R.D. Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): Respiratory toxicity, organ pathologies, and other physiological effects. Aquat. Toxicol. 2007, 82, 94–109. [Google Scholar] [CrossRef]
- Handy, R.D.; von der Kammer, F.; Lead, J.R.; Hassellov, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef]
- Garner, K.L.; Keller, A.A. Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies. J. Nanoparticle Res. 2014, 16, 2503. [Google Scholar] [CrossRef]
- Taghavi, S.M.; Momenpour, M.; Azarian, M.; Ahmadian, M.; Souri, F.; Taghavi, S.A.; Sadeghain, M.; Karchani, M. Effects of Nanoparticles on the Environment and Outdoor Workplaces. Electron. Phys. 2013, 5, 706–712. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Optical, electrochemical and catalytic methods for in-vitro diagnosis using carbonaceous nanoparticles: A review. Microchim. Acta 2019, 186, 50. [Google Scholar] [CrossRef]
- Samiei, F.; Shirazi, F.H.; Naserzadeh, P.; Dousti, F.; Seydi, E.; Pourahmad, J. Correction to: Toxicity of multi-wall carbon nanotubes inhalation on the brain of rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 29699. [Google Scholar] [CrossRef]
- Xiang, Q.Q.; Wang, D.; Zhang, J.L.; Ding, C.Z.; Luo, X.; Tao, J.; Ling, J.; Shea, D.; Chen, L.Q. Effect of silver nanoparticles on gill membranes of common carp: Modification of fatty acid profile, lipid peroxidation and membrane fluidity. Environ. Pollut. 2020, 256, 113504. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Lojk, J.; Repas, J.; Veranič, P.; Bregar, V.B.; Pavlin, M. Toxicity mechanisms of selected engineered nanoparticles on human neural cells in vitro. Toxicology 2020, 432, 152364. [Google Scholar] [CrossRef]
- Mohd Javed, A.; Maqusood, A.; Hisham, A.; Salman, A. Toxicity Mechanism of Gadolinium Oxide Nanoparticles and Gadolinium Ions in Human Breast Cancer Cells. Curr. Drug Metab. 2019, 20, 907–917. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Nature 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed. Res. Int. 2013, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Siddiqui, H.; Patil, G.; Ashquin, M.; Ahmad, I. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology 2010, 276, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, T. Progress in Genotoxicity Evaluation of Engineered Nanomaterials, 1st ed.; Larramendy, M.L., Ed.; Londres: London, UK, 2015. [Google Scholar] [CrossRef]
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Bio-interactions and risks of engineered nanoparticles. Environ. Res. 2019, 172, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Identification of Soil Bacteria Susceptible to TiO2and ZnO Nanoparticles. Appl. Environ. Microbiol. 2012, 78, 6749–6758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Cheng, S.; Singh, S. Oxidative stress-mediated genotoxic effect of zinc oxide 40 nanoparticles on Deinococcus radiodurans. 3 Biotech 2020, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 nanoparticles to Escherichia coli: Effects of particle size, crystal phase and water chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef] [Green Version]
- Frenk, S.; Ben-Moshe, T.; Dror, I.; Berkowitz, B.; Minz, D. Effect of Metal Oxide Nanoparticles on Microbial Community Structure and Function in Two Different Soil Types. PLoS ONE 2013, 8, 84441. [Google Scholar] [CrossRef] [Green Version]
- Lopes, I.; Ribeiro, R.; Antunes, F.E.; Rocha-Santos, T.A.; Rasteiro, M.G.; Soares, A.M.; Gonçalves, F.; Pereira, R. Toxicity and genotoxicity of organic and inorganic nanoparticles to the bacteria Vibrio fischeri and Salmonella typhimurium. Ecotoxicology 2012, 21, 637–648. [Google Scholar] [CrossRef]
- Schlich, K.; Hund-Rinke, K. Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils. Environ. Pollut. 2015, 196, 321–330. [Google Scholar] [CrossRef]
- Beddow, J.; Stolpe, B.; Cole, P.; Lead, J.R.; Sapp, M.; Lyons, B.P.; Colbeck, I.; Whitby, C. Effects of engineered silver nanoparticles on the growth and activity of ecologically important microbes. Environ. Microbiol. Rep. 2014, 6, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Duong, T.T.; Le, T.S.; Tran, T.T.; Nguyen, T.K.; Ho, C.T.; Dao, T.H.; Le, T.P.; Nguyen, H.C.; Dang, D.K.; Le, T.T.; et al. Inhibition effect of engineered silver nanoparticles to bloom forming cyanobacteria. Adv. Nat. Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Xu, S.; Yang, Y.; Li, L.; Chen, S.; Xu, A.; Wu, L. Insights into the Ecotoxicity of Silver Nanoparticles Transferred from Escherichia coli to Caenorhabditis elegans. Sci. Rep. 2016, 6, 36465. [Google Scholar] [CrossRef] [PubMed]
- Evariste, L.; Mottier, A.; Lagier, L.; Cadarsi, S.; Barret, M.; Sarrieu, C.; Soula, B.; Mouchet, F.; Flahaut, E.; Cels-Pinelli, C.; et al. Assessment of graphene oxide ecotoxicity at several trophic levels using aquatic microcosms. Carbon NY 2020, 156, 261–271. [Google Scholar] [CrossRef]
- Casado, M.P.; Macken, A.; Byrne, H.J. Ecotoxicological assessment of silica and polystyrene nanoparticles assessed by a multitrophic test battery. Environ. Int. 2013, 51, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.; Casey, A.; Tenuta, T.; Lynch, I.; Dawson, K.; Byrne, H.; Davoren, M. Preparation, characterization and ecotoxicological evaluation of four environmentally relevant species of N-isopropylacrylamide and N-isopropylacrylamide-co-N-tert-butylacrylamide copolymer nanoparticles. Aquat. Toxicol. 2009, 92, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant. Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere 2010, 81, 1253–1262. [Google Scholar] [CrossRef]
- Matranga, V.; Corsi, I. Toxic effects of engineered nanoparticles in the marine environment: Model organisms and molecular approaches. Mar. Environ. Res. 2012, 76, 32–40. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L.; Wang, S. Corrigendum: Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant. Sci. 2016, 7, 559. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.; Brice, D.; Brown, M.T. Interactions of silver nanoparticles with the marine macroalga, Ulva lactuca. Ecotoxicology 2012, 21, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Jiang, F.; Ma, C.; Rui, Y.; Rui, M.; Adeel, M.; Cao, W.; Xing, B. Alteration of Crop Yield and Quality of Wheat upon Exposure to Silver Nanoparticles in a Life Cycle Study. J. Agric. Food Chem. 2018, 66, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Verneuil, L.; Silvestre, J.; Randrianjatovo, I.; Marcato-Romain, C.E.; Girbal-Neuhauser, E.; Mouchet, F.; Flahaut, E.; Gauthier, L.; Pinelli, E. Double walled carbon nanotubes promote the overproduction of extracellular protein-like polymers in Nitzschia palea: An adhesive response for an adaptive issue. Carbon NY 2015, 88, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
- Rui, M.; Ma, C.; Tang, X.; Yang, J.; Jiang, F.; Pan, Y.; Xiang, Z.; Hao, Y.; Rui, Y.; Cao, W.; et al. Phytotoxicity of silver nanoparticles to peanut (Arachishypogaea L.): Physiological responses and food safety. Chem. Eng. 2017, 5, 6557–6567. [Google Scholar]
- Rui, M.; Ma, C.; White, J.C.; Hao, Y.; Wang, Y.; Tang, X.; Yang, J. Metal oxide nanoparticles alter peanut (Arachishypogaea L.) physiological response and reduce nutritional quality: A life cycle study. Environ. Sci. Nano 2018, 5, 2088–2102. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [Green Version]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Unrine, J.M.; Tsyusko, O.V.; Hunyadi, S.E.; Judy, J.D.; Bertsch, P.M. Effects of Particle Size on Chemical Speciation and Bioavailability of Copper to Earthworms (Eisenia fetida) Exposed to Copper Nanoparticles. J. Environ. Qual. 2010, 39, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; O’Carroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D.; et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.W.; Reinardy, H.C.; Shaw, B.J.; Henry, T.B.; Handy, R.D. Dietary toxicity of single-walled carbon nanotubes and fullerenes (C60) in rainbow trout (Oncorhynchus mykiss). Nanotoxicology 2011, 5, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.; Leung, P.T.; Djurisić, A.B.; Leung, K.M. Toxicities of nano zinc oxide to five marine organisms: Influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010, 396, 609–618. [Google Scholar] [CrossRef]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Canonico, B.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Saddick, S.; Afifi, M.; Zinada, O.A. Effect of Zinc nanoparticles on oxidative stress related genes and antioxidant enzymes activity in the brain of Oreochromisniloticus and Tilapia zillii. Saudi J. Biol. Sci 2015, 24, 1672–1678. [Google Scholar] [CrossRef]
- Salari Joo, H.; Kalbassi, M.R.; Yu, I.J.; Lee, J.H.; Johari, S.A. Bioaccumulation of silver nanoparticles in rainbow trout (Oncorhynchus mykiss): Influence of concentration and salinity. Aquat. Toxicol. 2013, 140–141, 398–406. [Google Scholar] [CrossRef]
- Wehmas, L.C.; Anders, C.; Chess, J.; Punnoose, A.; Pereira, C.B.; Greenwood, J.A.; Tanguay, R.L. Comparative Metal Oxide Nanoparticle Toxicity Using Embryonic Zebrafish. Toxicol. Rep. 2015, 2, 702–715. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhu, X.; Zhang, X.; Zhao, Z.; Liu, H.; George, R.; Wilson-Rawls, J.; Chang, Y.; Chen, Y. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 2011, 83, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.; Demir, V.; Adiguzel, R.; Arslan, Z. Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in gold fish (Carassiusauratus). J. Nanomater. 2013, 1, 6. [Google Scholar]
- Jovanović, B.; Whitley, E.M.; Kimura, K.; Crumpton, A.; Palić, D. Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens. Environ. Pollut. 2015, 203, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottier, A.; Mouchet, F.; Pinelli, É.; Gauthier, L.; Flahaut, E. Environmental impact of engineered carbon nanoparticles: From releases to effects on the aquatic biota. Curr. Opin. Biotechnol. 2017, 46, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.; Gupta, H.; Singh, D.; Mohanty, I.R.; Maheswari, U.; Vanage, G.; Joshi, D.S. Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (Chronic study) of repeated oral administration. J. Nanobiotechnol. 2014, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Di Bona, K.R.; Xu, Y.; Ramirez, P.A.; DeLaine, J.; Parker, C.; Bao, Y.; Rasco, J.F. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod. Toxicol. 2014, 50, 36–42. [Google Scholar] [CrossRef]
- Chen, R.; Ling, D.; Zhao, L.; Wang, S.; Liu, Y.; Bai, R.; Baik, S.; Zhao, Y.; Chen, C.; Hyeon, T. Parallel Comparative Studies on Mouse Toxicity of Oxide Nanoparticle- and Gadolinium-Based T1 MRI Contrast Agents. ACS Nano 2015, 9, 12425–12435. [Google Scholar] [CrossRef]
- Helal Neto, E.; Barros, A.; Saldanha-Gama, R.; Machado Brandão Costa, R.; Rebelo, L.; Alencar, R.; Costa, C.; Dos Santos, C.C.; Martínez-Máñez, R.; Ricci-Junior, E.; et al. Molecular and Cellular Risk Assessment of Healthy Human Cells and Cancer Human Cells Exposed to Nanoparticles. Int. J. Mol. Sci. 2019, 21, 230. [Google Scholar] [CrossRef] [Green Version]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz-Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [Green Version]
- Gea, M.; Bonetta, S.; Iannarelli, L.; Giovannozzi, A.M.; Maurino, V.; Bonetta, S.; Hodoroaba, V.D.; Armato, C.; Rossi, A.M.; Schilirò, T. Shape-engineered titanium dioxide nanoparticles (TiO(2)-NPs): Cytotoxicity and genotoxicity in bronchial epithelial cells. Food Chem. Toxicol. 2019, 127, 89–100. [Google Scholar] [CrossRef]
- Labrador-Rached, C.J.; Browning, R.T.; Braydich-Stolle, L.K.; Comfort, K.K. Toxicological Implications of Platinum Nanoparticle Exposure: Stimulation of Intracellular Stress, Inflammatory Response, and Akt Signaling In Vitro. J. Toxicol. 2018, 2018, 1367801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Zhong, G.; Chen, C.; Zhou, M.; Kline, D.J.; Jacob, R.J.; Xie, H.; He, S.; Huang, Z.; Dai, J.; et al. Uniform, Scalable, High-Temperature Microwave Shock for Nanoparticle Synthesis through Defect Engineering. Matter 2019, 1, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Y.; Nie, A.; Lu, A.; Jacob, R.J.; Gao, T.; Song, J.; Dai, J.; Wan, J.; Pastel, G.; et al. In Situ, Fast, High-Temperature Synthesis of Nickel Nanoparticles in Reduced Graphene Oxide Matrix. Adv. Energy Mater. 2017, 7, 1601783. [Google Scholar] [CrossRef]
- Xing, L.; ten Brink, G.H.; Chen, B.; Schmidt, F.P.; Haberfehlner, G.; Hofer, F.; Kooi, B.J.; Palasantzas, G. Synthesis and morphology of iron–iron oxide core–shell nanoparticles produced by high pressure gas condensation. Nanotechnology 2016, 27, 215703. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Chapter 5 Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Sifontes, Á.B. Biosíntesis de nanomateriales: Hacia el avance de la nanotecnología verde. Mundo Nano Rev. Interdiscip. Nanocienc. Nanotecnol. 2015, 7, 56–68. [Google Scholar] [CrossRef]
- Pandey, G. Prospects of Nanobioremediation in Environmental Cleanup. Orient. J. Chem. 2018, 34, 2838–2850. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–50107. [Google Scholar] [CrossRef] [Green Version]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Thorat, N.D.; Pawar, S.H. Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2012, 13, 466–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Chandrasekaran, R.; Gnanasekar, S. Antimicrobial and larvicidal activity of eco-friendly silver nanoparticles synthesized from endophytic fungi phomopsis liquidambaris. Biocatal. Agric. Biotechnol. 2018, 16, 22–30. [Google Scholar] [CrossRef]
- Usha Rani, P.; Rajasekharreddy, P. Green synthesis of silver-protein (core–shell) nanoparticles using Piper betle L. leaf extract and its ecotoxicological studies on Daphnia magna. Colloids Surfaces A Physicochem. Eng. Aspects 2011, 389, 188–194. [Google Scholar] [CrossRef]
- Rajan, R.; Chandran, K.; Harper, S.L. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crops Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Salah Eddine, L.; Abderrhmane, B.; Alonso-González, M.; Guerrero, A.; Romero, A. Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain. Chem. Pharm. 2020, 17, 100280. [Google Scholar] [CrossRef]
- Weijie, M.; Chongnv, W.; Xuming, P.; Weixin, J.; Yuhang, W.; Benhui, S. TiO 2 nanoparticles and multi-walled carbon nanotubes monitoring and bioremediation potential using ciliates Pseudocohnilembus persalinus. Ecotoxicol. Environ. Saf. 2020, 187, 109825. [Google Scholar] [CrossRef]
- Meier, S.; Moore, F.; Morales, A.; González, M.E.; Seguel, A.; Meriño-Gergichevich, C.; Rubilar, O.; Cumming, J.; Aponte, H.; Alarcón, D.; et al. Synthesis of calcium borate nanoparticles and its use as a potential foliar fertilizer in lettuce (Lactuca sativa) and zucchini (Cucurbita pepo). Plant. Physiol. Biochem. 2020, 151, 673–680. [Google Scholar] [CrossRef]
- Medina-Pérez, G.; Fernández-Luqueño, F.; Vazquez-Nuñez, E.; López-Valdez, F.; Prieto-Mendez, J.; Madariaga-Navarrete, A.; Miranda-Arámbula, M. Remediating Polluted Soils Using Nanotechnologies: Environmental Benefits and Risks. Polish J. Environ. Stud. 2019, 28, 1013–1030. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Fattal, E.; Nicolas, J. From poly(alkyl cyanoacrylate) to squalene as core material for the design of nanomedicines. J. Drug Target 2019, 27, 470–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, Y.; Ahmad, A.; Zein, S.; Tan, S. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Kemp, K.C.; Seema, H.; Saleh, M.; Le, N.H.; Mahesh, K.; Chandra, V.; Kim, K.S. Environmental applications using graphene composites: Water remediation and gas adsorption. Nanoscale 2013, 5, 3149–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diallo, M.; Fromer, N.; Jhon, M. Nanotechnology for sustainable development: Retrospective and outlook. J. Nanoparticle Res. 2013, 15, 2044. [Google Scholar] [CrossRef] [Green Version]
- Echiegu, E. Nanotechnology as a Tool for Enhanced Renewable Energy Application in Developing Countries. J. Fundam. Renew. Energy Appl. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mottaleb, M.; Byrne, J.; Chakarov, D. Nanotechnology and Solar Energy. Int. J. Photoenergy 2011, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, Z.; Khan, J.; LaGasse, M.K.; Suslick, K.S. Ultrasensitive Monitoring of Museum Airborne Pollutants Using a Silver Nanoparticle Sensor Array. ACS Sens. 2020. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Q.; Meng, G.; Wang, X.; Hu, X.; Han, F.; Lei, Y. Silver nanoparticle-assembled micro-bowl arrays for sensitive SERS detection of pesticide residue. Nanotechnology 2020, 31, 205303. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, T.; Liu, Q.; Liu, Z.; Li, D. Nanoparticle and microorganism detection with a side-micron-orifice-based resistive pulse sensor. Analyst 2020. [Google Scholar] [CrossRef]
- Singh, R. Prospects of Nanobiomaterials for Biosensing. Int. J. Electrochem. 2011, 2011, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. Available online: https://www.fda.gov/ (accessed on 11 December 2020).
- Wheeler, A. United States Environmental Protection Agency. Available online: https://www.epa.gov/ (accessed on 11 December 2020).
- Resnik, D.B. How should engineered nanomaterials be regulated for public and environmental health? AMA J. Ethics 2019, 21, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.F.; Baun, A.; Ganzleben, C. Nanomaterials and the European Water Framework Directive. Eur. J. Law Technol. 2011, 2, 1–11. [Google Scholar]
- Nanodatabase. Available online: https://nanodb.dk/en/ (accessed on 11 December 2020).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2021, 14, 166. https://doi.org/10.3390/ma14010166
Martínez G, Merinero M, Pérez-Aranda M, Pérez-Soriano EM, Ortiz T, Villamor E, Begines B, Alcudia A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials. 2021; 14(1):166. https://doi.org/10.3390/ma14010166
Chicago/Turabian StyleMartínez, Guillermo, Manuel Merinero, María Pérez-Aranda, Eva María Pérez-Soriano, Tamara Ortiz, Eduardo Villamor, Belén Begines, and Ana Alcudia. 2021. "Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review" Materials 14, no. 1: 166. https://doi.org/10.3390/ma14010166