Nanofibrillated Cellulose Surface Modification: A Review
Abstract
:1. Introduction
2. NFC Process Manufacturing
2.1. Sources

2.2. Devices



2.3. Pre-Treatments

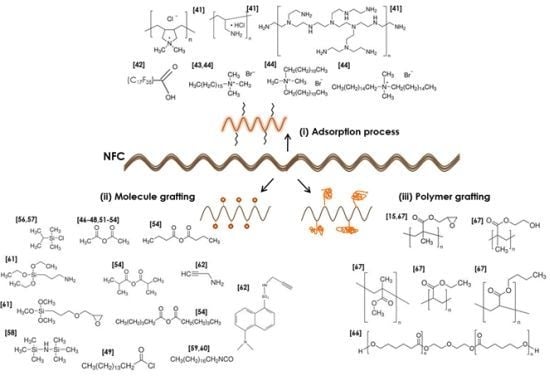
3. NFC Surface Modifications Strategies

3.1. Surface Adsorption on NFC
3.2. Molecule Chemical Grafting

3.3. Polymer Grafting
| Source of cellulose | Pretreatment | Reagent | Solvent/Process | DS * | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Surface adsorption | |||||||||
| Sulfite softwood dissolving pulp | Carboxy-methylation | Poly-DADMAC/ PEI/PHA | LbL assembly | Surf.Charge 515 µeq/g | [41] | ||||
| Sulfite softwood dissolving pulp | Carboxy-methylation | Perfluoro-octadecanoic acid | Coating on films | nd. | [42] | ||||
| Softwood bleached Kraft pulp | TEMPO oxidation | CTAB surfactant | Coating on films | nd. | [43] | ||||
| Softwood bleached Kraft pulp | TEMPO oxidation | CTAB/DDDAB/DHDAB surfactant | Mixing | 0.08–0.27 | [44] | ||||
| Molecule chemical grafting | |||||||||
| Bacterial Cellulose | Acetobacter xylinum | Acetic anhydride | Acetic acid + toluene | 0.04–2.77 | [46] | ||||
| Bacterial Cellulose | nc. | Acetic anhydride | No solvent | nd. | [48] | ||||
| Bacterial Cellulose | Acetobacter xylinum | Acetic anhydride | Acetic acid + toluene | 0.15–1.76 | [47] | ||||
| Bacterial Cellulose | Nata de coco | Palmitoyl acid | Gas phase | 1.47–2.01 | [49] | ||||
| Bleached sulphite wood pulp | nc. (Supplied by Borregaard) | Acetic anhydride | DMF | %Ac. 1.5–17 | [51] | ||||
| Kenaf Bast Fibers | Acetylation | Acetic anhydride | Pyridine | 1.07 | [52] | ||||
| Norway Spruce Kraft Pulp | No | Acetic anhydride | Toluene | 0.56–0.91 | [53] | ||||
| Sweden Domsjö Pulp | Enzyme | Acetic/Butyric/ Iso-butyric/Hexanoic anhydride | bmimPF6 Ionic liquid | 0.3/0.3/0.2/0.3 | [54] | ||||
| Sugar Beet Pulp | No | Isopropyl dimethylchlorosilane | Toluene | DSS = 0.025–0.36 | [55] | ||||
| Bleached Spruce Sulfite Cellulose | nc. (Supplied by Borregaard) | Chlorodimethyl isopropylsilane | Toluene | DSS = 0–0.16 | [56] | ||||
| Bleached Spruce Sulfite Cellulose | nc. (Supplied by Borregaard) | Chlorodimethyl isopropylsilane | Methanol water | n.c | [57] | ||||
| Kraft Pulp | nc. (Supplied by Daicel) | APS or GPS | Acetone | n.c | [61] | ||||
| Bleached Spruce Sulfite Cellulose | n.c | Hexamethyl disilazane | DMA or Toluene | n.c | [58] | ||||
| Bleached Sisal fibers | n.c | n-octadecyl isocyanate | Toluene | 0.09 | [59] | ||||
| Bleached Eucalyptus fibers | Enzyme | n-octadecyl isocyanate | Toluene | 0.09 | [60] | ||||
| Molecule chemical grafting | |||||||||
| Bleached Birch pulp | nc. (Supplied by Finnish center) | propargyl amine or 5-(dimethylamino)-N-(2-propyl)-1-naphtha lenesulfonamide | Water | 0.013 0.014 | [62] | ||||
| Polymer grafting | |||||||||
| Bleached Spruce Sulfite Cellulose | n.c | Cerium-induced GMA | Water + HNO3 | n.c | [15] | ||||
| Bleached Birch Pulp | n.c | Cerium-induced GMA EA MMA BuA, HEMA | Water + HNO3 | Graft yield 96%–99% 81%–85% 56%–75% 86%–89% 18%–63% | [67] | ||||
| Bleached sulfite softwood dissolving pulp (Domsjö) | Carboxy-methylation (DS = 0.089) | PCL-Sn(Oct)2-catalyzed ROP | Toluene | 16%–19%–21% | [66] | ||||
4. Conclusions
References
- Belgacem, M.N.; Gandini, A. Natural fiber-surface modification and characterisation. In Natural Fibre Reinforced Polymer Composites: From Macro to Nanoscale; Old City Publishing: Philadelphie, PA, USA, 2009; pp. 14–46. [Google Scholar]
- Belgacem, M.N.; Gandini, A. Cellulose-based composites and nanocomposites. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, the Netherlands, 2008; pp. 401–418. [Google Scholar]
- Belgacem, M.N.; Gandini, A. The surface modification of cellulose fibres for use as reinforcing elements in composite materials. Compos. Interfaces 2005, 12, 41–75. [Google Scholar]
- Bilodeau, M. Potential applications of nanofibrillated cellulose in printing and writing papers. In Proceedings of the 2012 TAPPI International Conference on Nanotechnology for Renewable Materials, Montreal, QC, Canada, 4–7 June 2012.
- Ensor, D.; Nieh, W.L.-S. INSCC workshop on international standards for cellulose nanomaterials. In Proceedings of the 2012 TAPPI International Conference on Nanotechnology for Renewable Materials, Montreal, QC, Canada, 4–7 June 2012.
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci. 1983, 28, 797–813. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. 1983, 28, 815–827. [Google Scholar]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H.; Nogi, M. Optically transparent composites reinforced with plant fiber-based nanofibers. Appl. Phys. A Mater. 2005, 81, 1109–1112. [Google Scholar]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-Catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar]
- Spence, K.L.; Venditti, R.A.; Habibi, Y.; Rojas, O.J.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Mechanical processing and physical properties. Bioresour. Technol. 2010, 101, 5961–5968. [Google Scholar]
- Taipale, T.; Österberg, M.; Nykänen, A.; Ruokolainen, J.; Laine, J. Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength. Cellulose 2010, 17, 1005–1020. [Google Scholar]
- Ahola, S.; Österberg, M.; Laine, J. Cellulose nanofibrils—Adsorption with poly(amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive. Cellulose 2008, 15, 303–314. [Google Scholar]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar]
- Stenstad, P.; Andresen, M.; Tanem, B.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar]
- Dinand, E.; Maureaux, A.; Chanzy, H.; Vincent, I.; Vignon, M.R. Microfibrillated Cellulose and Process for Making the Same from Vegetable Pulps Having Primary Walls, Especially from Sugar Beet Pulp. Eur. Pat. 1996. [Google Scholar]
- Habibi, Y.; Vignon, M. Optimization of cellouronic acid synthesis by TEMPO-mediated oxidation of cellulose III from sugar beet pulp. Cellulose 2008, 15, 177–185. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulose whiskers versus microfibrils: Influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 2008, 10, 425–432. [Google Scholar]
- Bhattacharya, D.; Germinario, L.T.; Winter, W.T. Isolation, preparation and characterization of cellulose microfibers obtained from bagasse. Carbohydr. Polym. 2008, 73, 371–377. [Google Scholar]
- Bendahou, A.; Kaddami, H.; Dufresne, A. Investigation on the effect of cellulosic nanoparticles’ morphology on the properties of natural rubber based nanocomposites. Eur. Polym. J. 2010, 46, 609–620. [Google Scholar]
- Siqueira, G.; Tadokoro, S.; Mathew, A.P.; Oksman, K. Carrot Nanofibers vs. Wood Pulp Nanofibers: Morphological and Mechanical Properties. In Proceedings of the 2010 TAPPI International Conference on Nanotechnology for the Forest Product Industry, Espoo, Finland, 27–29 September 2010.
- Siqueira, G.; Bras, J.; Dufresne, A. Luffa cylindrica as a lignocellulosic source of fiber, microfibrillated cellulose, and cellulose nanocrystals. BioResources 2010, 5, 727–740. [Google Scholar]
- Rodionova, G.; Saito, T.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø.; Fukuzumi, H.; Isogai, A. Mechanical and oxygen barrier properties of films prepared from fibrillated dispersions of TEMPO-oxidized Norway spruce and Eucalyptus pulps. Cellulose 2012, 19, 705–711. [Google Scholar]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar]
- Heiskanen, I.; Backfolk, K.; Vehviläinen, M.; Kamppuri, T.; Nousiainen, P. Process for Producing Microfibrillated Cellulose. Eur. Pat. 2012. [Google Scholar]
- Heiskanen, I.; Harlin, A.; Backfolk, K.; Laitinen, R. Process for production of microfibrillated cellulose in an extruder and microfibrillated cellulose produced according to the process. US Patent 20120214979, 26 October 2010. [Google Scholar]
- Walker, L.P.; Wilson, D.B. Enzymatic hydrolysis of cellulose: An overview. Bioresour. Technol. 1991, 36, 3–14. [Google Scholar]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; da Silva Perez, D.; Dufresne, A. Morphological investigation of nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2010, 17, 1147–1158. [Google Scholar]
- Siqueira, G.; Tapin-Lingua, S.; Bras, J.; da Silva Perez, D.; Dufresne, A. Mechanical properties of natural rubber nanocomposites reinforced with cellulosic nanoparticles obtained from combined mechanical shearing, and enzymatic and acid hydrolysis of sisal fibers. Cellulose 2011, 18, 57–65. [Google Scholar]
- Saito, T.; Okita, Y.; Nge, T.T.; Sugiyama, J.; Isogai, A. TEMPO-mediated oxidation of native cellulose: Microscopic analysis of fibrous fractions in the oxidized products. Carbohydr. Polym. 2006, 65, 435–440. [Google Scholar]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar]
- Isogai, T.; Saito, T.; Isogai, A. Wood cellulose nanofibrils prepared by TEMPO electro-mediated oxidation. Cellulose 2011, 18, 421–431. [Google Scholar]
- Sbiai, A.; Kaddami, H.; Sautereau, H.; Maazouz, A.; Fleury, E. TEMPO-mediated oxidation of lignocellulosic fibers from date palm leaves. Carbohydr. Polym. 2011, 86, 1445–1450. [Google Scholar]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71. [Google Scholar]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar]
- Aulin, C.; Johansson, E.; Wågberg, L.; Lindström, T. Self-Organized films from cellulose I nanofibrils using the layer-by-layer technique. Biomacromolecules 2010, 11, 872–882. [Google Scholar]
- Okahisa, Y.; Yoshida, A.; Miyaguchi, S.; Yano, H. Optically transparent wood–cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Compos. Sci. Technol. 2009, 69, 1958–1961. [Google Scholar]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar]
- Wagberg, L.; Decher, G.; Norgren, M.; Lindstrom, T.; Ankerfors, M.; Axnas, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar]
- Aulin, C.; Shchukarev, A.; Lindqvist, J.; Malmström, E.; Wågberg, L.; Lindström, T. Wetting kinetics of oil mixtures on fluorinated model cellulose surfaces. J. Colloid Interf. Sci. 2008, 317, 556–567. [Google Scholar]
- Syverud, K.; Xhanari, K.; Chinga-Carrasco, G.; Yu, Y.; Stenius, P. Films made of cellulose nanofibrils: Surface modification by adsorption of a cationic surfactant and characterization by computer-assisted electron microscopy. J. Nanopart. Res. 2011, 13, 773–782. [Google Scholar]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Reduction of water wettability of nanofibrillated cellulose by adsorption of cationic surfactants. Cellulose 2011, 18, 257–270. [Google Scholar]
- Martins, N.; Freire, C.; Pinto, R.; Fernandes, S.; Pascoal Neto, C.; Silvestre, A.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 2012, 19, 1425–1436. [Google Scholar]
- Kim, D.-Y.; Nishiyama, Y.; Kuga, S. Surface acetylation of bacterial cellulose. Cellulose 2002, 9, 361–367. [Google Scholar]
- Ifuku, S.; Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Yano, H. Surface modification of bacterial cellulose nanofibers for property enhancemnt of optically transparent composites: Dependence on acetyl-group DS. Biomacromolecules 2007, 8, 1973–1978. [Google Scholar]
- Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Ifuku, S.; Yano, H. Property enhancement of optically transparent bionanofiber composites by acetylation. Appl. Phys. Lett. 2006, 89, 233123:1–233123:3. [Google Scholar]
- Berlioz, S.; Molina-Boisseau, S.; Nishiyama, Y.; Heux, L. Gas-Phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 2009, 10, 2144–2151. [Google Scholar]
- Rodionova, G.; Lenes, M.; Eriksen, Ø.; Hoff, B.H; Gregersen, Ø.W. Surface modification of microfibrillated cellulose films by gas-phase esterification: Improvement of barrier properties. In Proceedings of the 2010 TAPPI International Conference on Nanotechnology for the Forest Product Industry, Espoo, Finland, 27–29 September 2010.
- Tingaut, P.; Zimmermann, T.; Lopez-Suevos, F. Synthesis and characterization of bionanocomposites with tunable properties from poly(lactic acid) and acetylated microfibrillated cellulose. Biomacromolecules 2010, 11, 454–464. [Google Scholar]
- Jonoobi, M.; Harun, J.; Mathew, A.; Hussein, M.; Oksman, K. Preparation of cellulose nanofibers with hydrophobic surface characteristics. Cellulose 2010, 17, 299–307. [Google Scholar]
- Rodionova, G.; Lenes, M.; Eriksen, Ø; Gregersen, Ø. Surface chemical modification of microfibrillated cellulose: Improvement of barrier properties for packaging applications. Cellulose 2011, 18, 127–134. [Google Scholar]
- Missoum, K.; Belgacem, M.N.; Barnes, J.-P.; Brochier-Salon, M.-C.; Bras, J. Nanofibrillated cellulose surface grafting in ionic liquid. Soft Matter 2012, 8, 8338–8349. [Google Scholar]
- Goussé, C.; Chanzy, H.; Cerrada, M.L.; Fleury, E. Surface silylation of cellulose microfibrils: Preparation and rheological properties. Polymer 2004, 45, 1569–1575. [Google Scholar]
- Andresen, M.; Johansson, L.; Tanem, B.; Stenius, P. Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 2006, 13, 665–677. [Google Scholar]
- Andresen, M.; Stenius, P. Water-in-oil emulsions stabilized by hydrophobized microfibrillated cellulose. J. Disper. Sci. Technol. 2007, 28, 837–844. [Google Scholar]
- Johansson, L.-S.; Tammelin, T.; Campbell, J.M.; Setälä, H.; Österberg, M. Experimental evidence on medium driven cellulose surface adaptation demonstrated using nanofibrillated cellulose. Soft Matter 2011, 7, 10917–10924. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 2010, 26, 402–411. [Google Scholar]
- Missoum, K.; Belgacem, N.; Bras, J. Organization of aliphatic chains grafted on nanofibrillated cellulose and influence on final properties. Cellulose 2012, 19, 1957–1973. [Google Scholar]
- Lu, J.; Askeland, P.; Drzal, L.T. Surface modification of microfibrillated cellulose for epoxy composite applications. Polymer 2008, 49, 1285–1296. [Google Scholar]
- Pahimanolis, N.; Hippi, U.; Johansson, L.-S.; Saarinen, T.; Houbenov, N.; Ruokolainen, J.; Seppälä, J. Surface functionalization of nanofibrillated cellulose using click-chemistry approach in aqueous media. Cellulose 2011, 18, 1201–1212. [Google Scholar]
- Filpponen, I.; Kontturi, E.; Nummelin, S.; Rosilo, H.; Kolehmainen, E.; Ikkala, O.; Laine, J. Generic method for modular surface modification of cellulosic materials in aqueous medium by sequential “Click” reaction and adsorption. Biomacromolecules 2012, 13, 736–742. [Google Scholar]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar]
- Lin, N.; Chen, G.; Huang, J.; Dufresne, A.; Chang, P.R. Effects of polymer-grafted natural nanocrystals on the structure and mechanical properties of poly(lactic acid): A case of cellulose whisker-graft-polycaprolactone. J. Appl. Polym. Sci. 2009, 113, 3417–3425. [Google Scholar]
- Lonnberg, H.; Larsson, K.; Lindström, T.; Hult, A.; Malmström, E. Synthesis of polycaprolactone-grafted microfibrillated cellulose for use in novel bionanocomposites–influence of the graft length on the mechanical properties. ACS Appl. Mater. Interfaces 2011, 3, 1426–1433. [Google Scholar]
- Littunen, K.; Hippi, U.; Johansson, L.-S.; Österberg, M.; Tammelin, T.; Laine, J.; Seppälä, J. Free radical graft copolymerization of nanofibrillated cellulose with acrylic monomers. Carbohydr. Polym. 2011, 84, 1039–1047. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745-1766. https://doi.org/10.3390/ma6051745
Missoum K, Belgacem MN, Bras J. Nanofibrillated Cellulose Surface Modification: A Review. Materials. 2013; 6(5):1745-1766. https://doi.org/10.3390/ma6051745
Chicago/Turabian StyleMissoum, Karim, Mohamed Naceur Belgacem, and Julien Bras. 2013. "Nanofibrillated Cellulose Surface Modification: A Review" Materials 6, no. 5: 1745-1766. https://doi.org/10.3390/ma6051745