Aminopropyl-Silica Hybrid Particles as Supports for Humic Acids Immobilization
Abstract
:1. Introduction
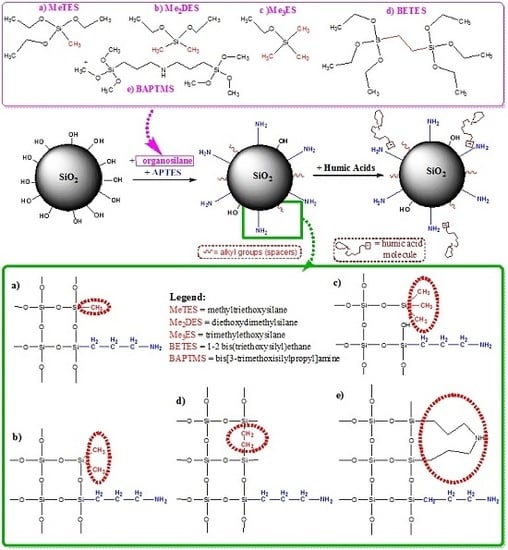
2. Results and Discussions
2.1. CHN Elemental Analysis (Determination of the Mass Fraction of Carbon, Hydrogen and Nitrogen)
| Samples | Silica Systems | Molar Ratio | Carbon (%) | Weight Loss 25–250 °C (%) | Weight Loss 250–450 °C (%) | Weight Loss 450–700 °C (%) | Inorganic Residue at 700 °C (%) |
|---|---|---|---|---|---|---|---|
| 1 | APTES | - | 27.63 | 23.1 | 7.5 | 22.1 | 47.3 |
| 2 | TEOS | - | 0.94 | 9.6 | 2.3 | 2.3 | 85.8 |
| 3 | TEOS/APTES | 20/1 | 5.98 | 11.0 | 4.4 | 4.1 | 80.5 |
| 4 | TEOS/APTES/MeTES | 20/1/1 | 3.27 | 9.6 | 3.7 | 2.9 | 83.8 |
| 5 | TEOS/APTES/Me2DES | 20/1/1 | 4.74 | 9.4 | 3.5 | 3.0 | 84.1 |
| 6 | TEOS/APTES/Me3ES | 20/1/1 | 3.83 | 11.0 | 3.6 | 3.2 | 82.2 |
| 7 | TEOS/APTES/BETES | 20/1/0.5 | 4.42 | 8.5 | 3.5 | 2.7 | 85.3 |
| 8 | TEOS/APTES/Me3ES | 10/1/1 | 5.01 | 13.6 | 4.6 | 5.1 | 76.7 |
| 9 | TEOS/BETES | 10/0.5 | 2.96 | 10.6 | 4.1 | 2.2 | 83.1 |
2.2. Thermogravimetric Analysis (TGA)

2.3. Particle Size Measurements (Dynamic Light Scattering (DLS) Technique)
| Samples | Silica Systems | Molar Ratio | Average Diameter (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| 1 | APTES | - | 580 | −19 |
| 2 | TEOS | - | 549 | −45 |
| 3 | TEOS/APTES | 20/1 | 691 | 15 |
| 4 | TEOS/APTES/MeTES | 20/1/1 | 1471 | 52 |
| 5 | TEOS/APTES/Me2DES | 20/1/1 | 858 | 27 |
| 6 | TEOS/APTES/Me3ES | 20/1/1 | 653 | 13 |
| 7 | TEOS/APTES/BETES | 20/1/0.5 | 716 | 53 |
| 8 | TEOS/APTES/Me3ES | 10/1/1 | 558 | −5 |
| 9 | TEOS/BETES | 10/0.5 | 590 | −48 |

2.4. Zeta Potential Measurements (Laser Doppler Velocimetry (LDV) Technique)

2.5. Environmental Scanning Electronic Microscopy (ESEM)

2.6. Fourier Transformed Infrared Spectroscopy (FTIR) Spectra

2.7. Solid State Nuclear Magnetic Resonance (ssNMR)

2.8. Thermogravimetric Analysis Coupled with Mass Spectroscopy (TG-MS)
2.9. Porosimetry


2.10. The Humic Acid (HA) Immobilization Tests of the Novel Aminosilica Supports


3. Experimental Section
3.1. Materials
3.2. Characterization Techniques
3.3. Synthesis of the Silica Hybrid Supports
- Step 1.
- Preparation of Pristine Silica Particles (Preformed SiO2 Particles)
- Step 2.
- Preparation of Amino-Functionalized Silica Particles (NH2-SiO2 Particles)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. NonCryst. Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; McCarthy, T.J. Self-assembly is not the only reaction possible between alkyltrichlorosilanes and sufaces: Mononuclear and oligomeric covalently attached layers of dichloro- and trichloroalkylsilanes on silicon. Langmuir 2000, 16, 7268–7274. [Google Scholar] [CrossRef]
- Fatunbi, H.O.; Bruch, M.D. Characterization of the structural morphology of chemically modified silica prepared by surface polymerization of a mixture of long and short alkyl chains using 13C and 29Si NMR spectroscopy. Langmuir 2013, 29, 4974–4987. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.G.S.; Miranda, B.S.; Dias, J.A. Attachment of two distinct humic acids into a silica gel surface. Colloids Surf. A 2004, 242, 137–143. [Google Scholar] [CrossRef]
- Szabo, G.; Angelova, A.; Guczi, J.; Bulman, R.A. An examination of the uptake of radioiodine by chemically bound humic acid and by some solid phase of soil. Sci. Total Environ. 1993, 130–131, 375–382. [Google Scholar] [CrossRef]
- Szabo, G.; Prosser, S.L.; Bulman, R.A. Determination of the adsorption coefficient (KOC) of some aromatics for soil by RP-HPLC on two immobilized humic acid phases. Chemosphere 1990, 21, 777–788. [Google Scholar] [CrossRef]
- Guczi, J.; Angelova, A.; Bulman, R.A.; Szabo, G. Investigation of the interactions of 110 mAg+ and 125I− with humic acid chemically immobilized on silica gel. React. Polym. 1992, 17, 61–68. [Google Scholar] [CrossRef]
- Koopal, L.K.; Yang, Y.; Minnaard, A.J.; Theunissen, P.L.M.; van Riemsdijk, W.H. Chemical immobilization of humic acid on silica. Colloids Surf. A 1998, 141, 385–395. [Google Scholar] [CrossRef]
- Yang, Y.H.; Koopal, L.K. Immobilisation of humic acids and binding of nitrophenol to immobilized humics. Colloids Surf. A 1999, 151, 201–212. [Google Scholar] [CrossRef]
- Armon, R.; Zolkov, C.H.; Laor, Y. Entrapment of humic acid in a sol-gel matrix—A new tool for sorption studies. J. Sol Gel Sci. Technol. 2000, 19, 95–100. [Google Scholar] [CrossRef]
- Klavins, M.; Eglite, L. Immobilisation of humic substances. Colloids Surf. A 2002, 203, 47–54. [Google Scholar] [CrossRef]
- De la Rosa, G.; Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; Herrera, I.; Contreras, C. Use of silica-immobilized humin for heavy metal removal from aqueous solution under flow conditions. Bioresour. Technol. 2003, 90, 11–17. [Google Scholar] [CrossRef]
- Helles, R. Wastewater COD removal using sol-gel immobilized humicacid. J. Toxicol. Environ. Health Sci. 2010, 2, 7–10. [Google Scholar]
- Rosa, A.H.; Vicente, A.A.; Rocha, J.C.; Trevisan, H.C. A new application of humic substances: Activation of supports for invertase immobilization. Fresenius J. Anal. Chem. 2000, 368, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Treccani, L.; Klein, T.Y.; Meder, F.; Pardun, K.; Rezwan, K. Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 2013, 9, 7115–7150. [Google Scholar] [CrossRef] [PubMed]
- Blaszykowski, C.; Sheikh, S.; Benvenuto, P.; Thomson, M. New functionalizable alkyltrichlorosiloxane surface modifiers for biosensor and biomedical applications. Langmuir 2012, 28, 2318–2322. [Google Scholar] [CrossRef] [PubMed]
- Leal, O.; Bolivar, C.; Ovalles, C.; Garcia, J.J.; Espidel, Y. Reversible adsorption of carbon dioxide on amine surface-bonded silica gel. Inorg. Chim. Acta 1995, 240, 183–189. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Yao, W.; Cen, W.; Wang, H.; Weng, X.; Wu, Z. The effects of surface acidity on CO2 adsorption over amine functionalized protonated titanate nanotubes. RSC Adv. 2013, 3, 18803–18810. [Google Scholar] [CrossRef]
- Bacsik, Z.; Atluri, R.; Garcia-Bennett, A.E.; Hedin, N. Temperature-induced uptake of CO2 and formation of carbamates in mesocaged silica modified with n-propylamines. Langmuir 2010, 26, 10013–10024. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, G.; Michel, D. High-Resolution Solid-State NMR of Silicates and Zeolites; John Wiley and Sons: Chichester, UK, 1994. [Google Scholar]
- Duer, M.J. Introduction to Solis-State NMR Spectroscopy; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Bonhomme, C.; Coelho, C.; Baccile, N.; Gervais, C.; Azais, T.; Babonneau, F. Advanced solid state NMR techniques for the characterization of sol-gel-derived materials. Acc. Chem. Res. 2007, 40, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, E.O.; Memory, J. High Resolution NMR in the Solid State; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Tabellen Zur Strukturaufklärung Organischer Verbindungen Mit Spektroskopischen Methoden; Springer-Verlag: Berlin, Germany, 1976. [Google Scholar]
- Cestari, A.R.; Vieira, E.F.S.; Vieira, G.S.; Almeida, L.E. Aggregation and adsorption of reactive dyes in the presence of an anionic surfactant on mesoporous aminopropyl silica. J. Colloid Interface Sci. 2007, 309, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Janos, P. Separation methods in the chemistry of humic substances. J. Chromatogr. A 2003, 983, 1–18. [Google Scholar] [CrossRef]
- Brambilla, R.; Pires, G.P.; dos Santos, J.H.Z.; Lacherda-Miranda, M.S.; Chornik, B. Octadecylsilane-modified silicas prepared by grafting and sol-gel methods. J. Electron. Spectrosc. 2007, 156–158, 413–420. [Google Scholar] [CrossRef]
- Jung, H.S.; Moon, D.S.; Lee, J.K. Quantitative analysis and efficient surface modification of silica nanoparticles. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Meera, K.M.S.; Sankar, R.M.; Paul, J.; Jaisankara, S.N.; Mandal, A.B. The influence of applied silica nanoparticles on a bio-renewable castor oil based polyurethane nanocomposite and its physicochemical properties. Phys. Chem. Chem. Phys. 2014, 16, 9276–9288. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sándor, M.; Nistor, C.L.; Szalontai, G.; Stoica, R.; Nicolae, C.A.; Alexandrescu, E.; Fazakas, J.; Oancea, F.; Donescu, D. Aminopropyl-Silica Hybrid Particles as Supports for Humic Acids Immobilization. Materials 2016, 9, 34. https://doi.org/10.3390/ma9010034
Sándor M, Nistor CL, Szalontai G, Stoica R, Nicolae CA, Alexandrescu E, Fazakas J, Oancea F, Donescu D. Aminopropyl-Silica Hybrid Particles as Supports for Humic Acids Immobilization. Materials. 2016; 9(1):34. https://doi.org/10.3390/ma9010034
Chicago/Turabian StyleSándor, Mónika, Cristina Lavinia Nistor, Gábor Szalontai, Rusandica Stoica, Cristian Andi Nicolae, Elvira Alexandrescu, József Fazakas, Florin Oancea, and Dan Donescu. 2016. "Aminopropyl-Silica Hybrid Particles as Supports for Humic Acids Immobilization" Materials 9, no. 1: 34. https://doi.org/10.3390/ma9010034