Commercial Applications of Metal Foams: Their Properties and Production
Abstract
:1. Introduction
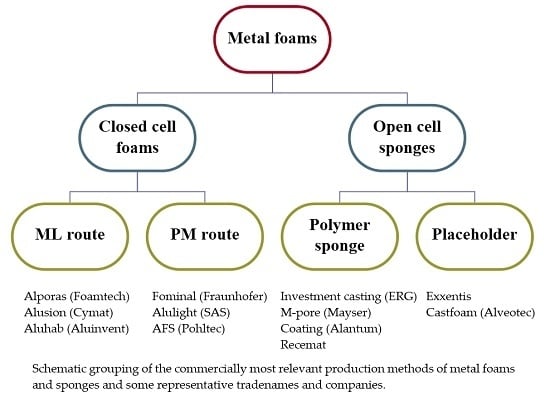
2. Commercial Production Procedures

2.1. Melt Metallurgical Route
2.2. Powder Metallurgical Route
2.3. Based on Polymer Sponge Structure
2.4. Based on Dissolution of a Placeholder
3. Properties
3.1. Mechanical Properties
3.2. Functional Properties

3.3. Other Properties
4. Costs and Feasibility Considerations
5. Applications
5.1. Structural Applications







5.2. Functional Applications





5.3. Architectural Applications





5.4. Design, Art and Decoration




6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Byakova, A.V.; Gnyloskurenko, S.V.; Nakamura, T.; Raychenko, O.I. Influence of wetting conditions on bubble formation at orifice in an inviscid liquid: Mechanism of bubble evolution. Colloids Surf. A 2003, 229, 19–32. [Google Scholar] [CrossRef]
- Mao, D.; Edwards, J.R.; Harvey, A. Prediction of foam growth and its nucleation in free and limited expansion. Chem. Eng. Sci. 2006, 61, 1836–1845. [Google Scholar] [CrossRef]
- Rack, A.; Helwig, H.M.; Bütow, A.; Rueda, A.; Matijašević-Lux, B.; Helfen, L.; Goebbels, J.; Banhart, J. Early pore formation in aluminium foams studied by synchrotron-based microtomography and 3-D image analysis. Acta Mater. 2009, 57, 4809–4821. [Google Scholar] [CrossRef]
- Asavavisithchai, S.; Kennedy, A.R. In-situ oxide stabilization development of aluminum foams in powder metallurgical route. High Temp. Mater. Processes 2011, 30, 113–120. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.; Mukherjee, M.; Jiménez, C.; Rack, A.; Banhart, J. Metal foaming investigated by X-ray radioscopy. Metals 2011, 2, 10–21. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zuo, X.Q.; Lu, J.S.; Zhou, Y. Bubble nucleation of PM Al-9Si foam. Adv. Mater. Res. 2011, 183–185, 1682–1686. [Google Scholar] [CrossRef]
- Asavavisithchai, S.; Kennedy, A.R. The role of oxidation during compaction on the expansion and stability of a foams made via a PM route. Adv. Eng. Mater. 2006, 8, 568–572. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Chen, X.; Liu, Y.; Fan, X. Foam stability in gas injection foaming process. J. Mater. Sci. 2010, 45, 6481–6493. [Google Scholar] [CrossRef]
- Vandewalle, N.; Caps, H.; Delon, G.; Saint-Jalmes, A.; Rio, E.; Saulnier, L.; Adler, M.; Biance, A.L.; Pitois, O.; Addad, S.C.; et al. Foam stability in microgravity. J. Phys. Conf. Ser. 2011, 327. [Google Scholar] [CrossRef]
- Körner, C.; Arnold, M.; Singer, R.F. Metal foam stabilization by oxide network particles. Mater. Sci. Eng. A 2005, 396, 28–40. [Google Scholar] [CrossRef]
- Mukherjee, M.; Garcia-Moreno, F.; Jiménez, C.; Banhart, J. Al and Zn foams blown by an intrinsic gas source. Adv. Eng. Mater. 2010, 12, 472–477. [Google Scholar] [CrossRef]
- Mukherjee, M.; Garcia-Moreno, F.; Banhart, J. Solidification of metal foams. Acta Mater. 2010, 58, 6358–6370. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.; Solorzano, E.; Banhart, J. Kinetics of coalescence in liquid aluminium foams. Soft Matter 2011, 7, 9216–9223. [Google Scholar] [CrossRef]
- Schäffler, P.; Hanko, G.; Mitterer, H.; Zach, P. Alulight Metal Foam Products. In Proceedings of the Porous Metals and Metallic Foams: Metfoam, Kyoto, Japan, 5–7 September 2007; Lefebvre, L.P., Banhart, J., Dunand, D.C., Eds.; The Japan Institute of Metals: Kyoto, Japan, 2008; pp. 7–10. [Google Scholar]
- Banhart, J.; Seeliger, H.-W. Recent trends in aluminum foam sandwich technology. Adv. Eng. Mater. 2012, 14, 1082–1087. [Google Scholar] [CrossRef]
- Banhart, J.; Baumeister, J.; Irretier, O.; Jöbstl, J. Cost-effective production techniques for the manufacture of aluminium foam. Aluminium 2000, 76, 491–496. [Google Scholar]
- Kevorkijan, M. Cost effective foaming with CaCO3. Aluminium 2010, 12, 59–65. [Google Scholar]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Scheffler, M.; Colombo, P. Cellular Ceramics: Structure, Manufacturing, Properties and Appliations; WILEY-VCH: Weinheim, Germany, 2005; p. 645. [Google Scholar]
- Davies, G.J.; Zhen, S. Metallic foams: Their production, properties and applications. J. Mater. Sci. 1983, 18, 1899–1911. [Google Scholar] [CrossRef]
- Nakajima, H. Fabrication, properties and application of porous metals with directional pores. Prog. Mater. Sci. 2007, 52, 1091–1173. [Google Scholar] [CrossRef]
- Kranzlin, N.; Niederberger, M. Controlled fabrication of porous metals from the nanometer to the macroscopic scale. Mater. Horiz. 2015, 2, 359–377. [Google Scholar] [CrossRef]
- Qin, J.; Chen, Q.; Yang, C.; Huang, Y. Research process on property and application of metal porous materials. J. Alloys Compd. 2016, 654, 39–44. [Google Scholar] [CrossRef]
- Lefebvre, L.P.; Banhart, J.; Dunand, D.C. Porous metals and metallic foams: Current status and recent developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Metalfoam.net. Available online: www.metalfoam.net (accessed on 27 July 2015).
- Ashby, M.F.; Evans, A.G.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Metal Foams: A Design Guide; Butterworth-Heinemann: Boston, MA, USA, 2000. [Google Scholar]
- Baumeister, J.; Weise, J. Metallic foams. In Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Kim, S.; Lee, C.-W. A review on manufacturing and application of open-cell metal foam. Proc. Mater. Sci. 2014, 4, 305–309. [Google Scholar] [CrossRef]
- Akiyama, S.; Ueno, H.; Imagawa, K.; Kitahara, A.; Nagata, S.; Morimoto, K.; Nishikawa, T.; Itoh, M. Foamed Metal and Method of Producing Same. U.S. Patent 4,713,277, 15 December 1987. [Google Scholar]
- Foamtech. Available online: http://www.foamtech.co.kr/eng02/ (accessed on 11 December 2015).
- Shanxi Putai. Available online: http://2014.cnputai.com/?en/ (accessed on 11 December 2015).
- Jin, I.; Kenny, L.D.; Sang, H. Method of Producing Lightweight Foamed Metal. U.S. Patent 4,973,358, 27 November 1990. [Google Scholar]
- Babcsan, N.; Beke, S.; Makk, P. Method of Producing a Metal Foam by Oscillations and thus Obtained Metal Foam Product. WO Patent 2010/064059 A2, 10 June 2010. [Google Scholar]
- Allen, B.C.; Mote, M.W.; Sabroff, A.M. Method of Making Foamed Metal. U.S. Patent 3,087,807, 30 April 1963. [Google Scholar]
- Asavavisithchai, S.; Kennedy, A.R. Effect of powder oxide content on the expansion and stability of PM-route Al foams. J. Colloid Interface Sci. 2006, 297, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, J. Method for Producing Porous Metal Bodies. German Patent 4,018,360, 29 May 1991. [Google Scholar]
- Duocel. Available online: http://www.ergaerospace.com/index.html (accessed on 11 December 2015).
- M-pore. Available online: http://www.m-pore.de/ (accessed on 24 July 2015).
- Paserin, V.; Marcuson, S.; Shu, J.; Wilkinson, D.S. CVD technique for inco nickel foam production. Adv. Eng. Mater. 2004, 6, 454–459. [Google Scholar] [CrossRef]
- Alantum. Available online: http://www.alantum.com/ (accessed on 14 December 2015).
- Recemat. Available online: http://www.recemat.nl/eng/ (accessed on 24 July 2015).
- Exxentis. Available online: http://www.exxentis.co.uk/ (accessed on 11 December 2015).
- Alveotec. Available online: http://www.alveotec.fr/en/ (accessed on 11 December 2015).
- Bram, M.; Stiller, C.; Buchkremer, H.P.; Stöver, D.; Bauer, H. Preparation and Characterization of High-Porosity Titanium, Stainless Steel, and Superalloy Parts. In Proceedings of the Metal Foams and Porous Metal Structures: Metfoam 1999, Bremen, Germany, 14–16 June 1999; Banhart, J., Ed.; MIT Publishing: Bremen, Germany, 1999; pp. 197–202. [Google Scholar]
- Wazen, R.M.; Lefebvre, L.-P.; Baril, E.; Nanci, A. Initial evaluation of bone ingrowth into a novel porous titanium coating. J. Biomed. Mater. Res. Part B 2010, 94B, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.; Rodríguez, J.A.; Arias, S.; Echeverry, M.; Robledo, S.; Amigo, V.; Pavón, J.J. Processing, characterization and biological testing of porous titanium obtained by space-holder technique. J. Mater. Sci. 2012, 47, 6565–6576. [Google Scholar] [CrossRef]
- Gibson, L.; Ashby, M. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Weaire, D.; Hutzler, S. The Physics of Foams; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Hall, I.W.; Guden, M.; Yu, C.-J. Crushing of aluminum closed cell foams: Density and strain rate effects. Scr. Mater. 2000, 43, 515–521. [Google Scholar] [CrossRef]
- Olurin, O.B.; Fleck, N.A.; Ashby, M.F. Deformation and fracture of aluminium foams. Mater. Sci. Eng. A 2000, 291, 136–146. [Google Scholar] [CrossRef]
- Kennedy, A.R. Aspects of the reproducibility of mechanical properties in Al based foams. J. Mater. Sci. 2004, 39, 3085–3088. [Google Scholar] [CrossRef]
- Ramamurty, U.; Paul, A. Variability in mechanical properties of a metal foam. Acta Mater. 2004, 52, 869–876. [Google Scholar] [CrossRef]
- Beals, J.T.; Thomson, M.S. Density gradient effects on aluminium foam compression behaviour. J. Mater. Sci. 1997, 32, 3595–3600. [Google Scholar] [CrossRef]
- Evans, A.G.; Hutchinson, J.W.; Ashby, M.F. Multifunctionality of cellular metal systems. Prog. Mater. Sci. 1998, 43, 171–221. [Google Scholar] [CrossRef]
- Sugimura, Y.; Rabiei, A.; Evans, A.G.; Harte, A.M.; Fleck, N.A. Compression fatigue of a cellular Al alloy. Mater. Sci. Eng. A 1999, 269, 38–48. [Google Scholar] [CrossRef]
- Harte, A.-M.; Fleck, N.A.; Ashby, M.F. Fatigue failure of an open cell and a closed cell aluminium alloy foam. Acta Mater. 1999, 47, 2511–2524. [Google Scholar] [CrossRef]
- Lehmhus, D.; Marschner, C.; Banhart, J.; Bomas, H. Influence of heat treatment on compression fatigue of aluminium foams. J. Mater. Sci. 2002, 37, 3447–3451. [Google Scholar] [CrossRef]
- Kolluri, M.; Mukherjee, M.; Garcia-Moreno, F.; Banhart, J.; Ramamurty, U. Fatigue of a laterally constrained closed cell aluminum foam. Acta Mater. 2008, 56, 1114–1125. [Google Scholar] [CrossRef]
- Harte, A.-M.; Fleck, N.A.; Ashby, M.F. The fatigue strength of sandwich beams with an aluminium alloy foam core. Int. J. Fatigue 2001, 23, 499–507. [Google Scholar] [CrossRef]
- Hanssen, A.G.; Langseth, M.; Hopperstad, O.S. Optimum design for energy absorption of square aluminium columns with aluminium foam filler. Int. J. Mech. Sci. 2001, 43, 153–176. [Google Scholar] [CrossRef]
- Ramachandra, S.; Kumar, P.S.; Ramamurty, U. Impact energy absorption in an Al foam at low velocities. Scr. Mater. 2003, 49, 741–745. [Google Scholar] [CrossRef]
- Körner, C. Integral Foam Molding of Light Metals: Technology, Foam Physics and Foam Simulation; Springer-Verlag: Berlin, Heidelberg, Germany, 2008. [Google Scholar]
- Hartmann, J.; Trepper, A.; Körner, C. Aluminum integral foams with near-microcellular structure. Adv. Eng. Mater. 2011, 13, 1050–1055. [Google Scholar] [CrossRef]
- Lehmhus, D.; Busse, M.; Chen, Y.; Bomas, H.; Zoch, H.W. Influence of core and face sheet materials on quasi-static mechanical properties and failure in aluminium foam sandwich. Adv. Eng. Mater. 2008, 10, 863–867. [Google Scholar] [CrossRef]
- Lies, C.; Hohlfeld, J.; Hipke, T. Adhesion in Sandwiches with Aluminum Foam Core. In Proceedings of the Porous Metals and Metallic Foams: Metfoam 2007, Montreal, QC, Canada, 5–7 September 2007; Lefebvre, L.P., Banhart, J., Dunand, D.C., Eds.; DEStech Publications, Inc: Montreal, QC, Canada, 2008; pp. 31–34. [Google Scholar]
- Han, F.; Seiffert, G.; Zhao, Y.; Gibbs, B. Acoustic absorption behaviour of an open-celled aluminium foam. J. Phys. D Appl. Phys. 2003, 36. [Google Scholar] [CrossRef]
- Banhart, J.; Baumeister, J.; Weber, M. Damping properties of aluminium foams. Mater. Sci. Eng. A 1996, 205, 221–228. [Google Scholar] [CrossRef]
- Avilova, G.M.; Grushin, A.E.; Lebedeva, I.V. Sound Insulation of Foam Shells. In Proceedings of the XIII Session of the Russian Acoustical Society, Moscow, Russia, 25–29 August 2003; pp. 879–881.
- Byakova, A.; Gnyloskurenko, S.; Bezimyanniy, Y.; Nakamura, T. Closed-cell aluminum foam of improved sound absorption ability: Manufacture and properties. Metals 2014, 4, 445–454. [Google Scholar] [CrossRef]
- Jiejun, W.; Chenggong, L.; Dianbin, W.; Manchang, G. Damping and sound absorption properties of particle reinforced Al matrix composite foams. Compos. Sci. Technol. 2003, 63, 569–574. [Google Scholar] [CrossRef]
- Kádár, C.; Chmelı́k, F.; Rajkovits, Z.; Lendvai, J. Acoustic emission measurements on metal foams. J. Alloys Compd. 2004, 378, 145–150. [Google Scholar] [CrossRef]
- Fiedler, T.; Belova, I.V.; Öchsner, A.; Murch, G.E. Non-linear calculations of transient thermal conduction in composite materials. Comput. Mater. Sci. 2009, 45, 434–438. [Google Scholar] [CrossRef]
- Fiedler, T.; Solorzano, E.; Garcia-Moreno, F.; Ochsner, A.; Belova, I.V.; Murch, G.E. Lattice Monte Carlo and experimental analyses of the thermal conductivity of random-shaped cellular aluminum. Adv. Eng. Mater. 2009, 11, 843–847. [Google Scholar] [CrossRef]
- Fiedler, T.; Solórzano, E.; Garcia-Moreno, F.; Öchsner, A.; Belova, I.V.; Murch, G.E. Computed tomography based finite element analysis of the thermal properties of cellular aluminium. Materialwiss. Werkstofftech. 2009, 40, 139–143. [Google Scholar] [CrossRef]
- Lu, T.J.; Stone, H.A.; Ashby, M.F. Heat transfer in open-cell metal foams. Acta Mater. 1998, 46, 3619–3635. [Google Scholar] [CrossRef]
- Lu, T.J.; Chen, C. Thermal transport and fire retardance properties of cellular aluminium alloys. Acta Mater. 1999, 47, 1469–1485. [Google Scholar] [CrossRef]
- Solórzano, E.; Reglero, J.A.; Rodríguez-Pérez, M.A.; Lehmhus, D.; Wichmann, M.; de Saja, J.A. An experimental study on the thermal conductivity of aluminium foams by using the transient plane source method. Int. J. Heat Mass Transfer 2008, 51, 6259–6267. [Google Scholar] [CrossRef]
- Golovin, I.S.; Sinning, H.R. Damping in some cellular metallic materials. J. Alloys Compd. 2003, 355, 2–9. [Google Scholar] [CrossRef]
- Testing of Metallic Materials—Compression Test of Metallic Cellular Materials; DIN 50134; Beuth Verlag: Berlin, Germany, 2008.
- Banhart, J.; Baumeister, J. Deformation characteristics of metal foams. J. Mater. Sci. 1998, 33, 1431–1440. [Google Scholar] [CrossRef]
- Rasooli, A.; Divandari, M.; Shahverdi, H.R.; Boutorabi, M.A. Kinetics and mechanism of titanium hydride powder and aluminum melt reaction. Int. J. Miner. Metall. Mater. 2012, 19, 165–172. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Rack, A.; Tucoulou, R.; Klaus, M.; Pfretzschner, B.; Rack, T.; Cloetens, P.; Banhart, J. Partial decomposition of TiH2 studied in situ by energy-dispersive diffraction and ex situ by diffraction microtomography of hard X-ray synchrotron radiation. Scr. Mater. 2012, 66, 757–760. [Google Scholar] [CrossRef]
- Cymat. Available online: http://www.cymat.com/ (accessed on 20 July 2015).
- Stöbener, K.; Baumeister, J.; Rausch, G.; Rausch, M. Forming metal foams by simpler methods for cheaper solutions. Met. Powder Rep. 2005, 60, 12–16. [Google Scholar] [CrossRef]
- Nakamura, T.; Gnyloskurenko, S.V.; Sakamoto, K.; Byakova, A.V.; Ishikawa, R. Development of new foaming agent for metal foam. Mater. Trans. 2002, 43, 1191–1196. [Google Scholar] [CrossRef]
- Gergely, V.; Curran, D.C.; Clyne, T.W. The FOAMCARP process: Foaming of aluminium MMCs by the chalk-aluminium reaction in precursors. Compos. Sci. Technol. 2003, 63, 2301–2310. [Google Scholar] [CrossRef]
- Bryant, D.; Wilhelmy, D.; Kallivayalil, J.; Wang, W. Development of aluminum foam processes and products. Mater. Sci. Forum 2006, 519–521, 1193–1200. [Google Scholar] [CrossRef]
- Haesche, M.; Lehmhus, D.; Weise, J.; Wichmann, M.; Mocellin, I.C.M. Carbonates as foaming agent in chip-based aluminium foam precursor. J. Mater. Sci. Technol. 2010, 26, 845–850. [Google Scholar] [CrossRef]
- Byakova, A.V.; Gnyloskurenko, S.V.; Sirko, A.I.; Milman, Y.V.; Nakamura, T. The role of foaming agent in structure and mechanical performance of Al based foams. Mater. Trans. 2006, 47, 2131–2136. [Google Scholar] [CrossRef]
- Kevorkijan, V.; Skapin, S.D.; Paulin, I.; Sustarsic, B.; Jenko, M. Synthesis and characterisation of closed cells aluminium foams containing dolomite powders as foaming agent. Mater. Tech. 2010, 44, 363–371. [Google Scholar]
- Papadopoulos, D.P.; Omar, H.; Stergioudi, F.; Tsipas, S.A.; Michailidis, N. The use of dolomite as foaming agent and its effect on the microstructure of aluminium metal foams—Comparison to titanium hydride. Colloids Surf. A 2011, 382, 118–123. [Google Scholar] [CrossRef]
- Byakova, A.; Kartuzov, I.; Gnyloskurenko, S.; Nakamura, T. The role of foaming agent and processing route in mechanical performance of fabricated aluminum foams. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef]
- Neugebauer, R.; Hipke, T. Machine tools with metal foams. Adv. Eng. Mater. 2006, 8, 858–863. [Google Scholar] [CrossRef]
- Pohltec metalfoam. Available online: http://metalfoam.de/ (accessed on 27 July 2015).
- IWU. Available online: http://www.iwu.fraunhofer.de/ (accessed on 27 July 2015).
- Baumeister, J.; Banhart, J.; Weber, M. Aluminium foams for transport industry. Mater. Des. 1997, 18, 217–220. [Google Scholar] [CrossRef]
- Evolution. Available online: http://evolutionproject.eu/ (accessed on 14 December 2015).
- Kretz, R.; Götzinger, B. Energy Absorbing Behaviour of Aluminium Foams: Head Impact Tests on an A-Pillar of a Passenger Car. In Proceedings of the Cellular Metals and Metal Foaming Technology: Metfoam, Bremen, Germany, 18–20 June 2001; Banhart, J., Ashby, M.F., Fleck, N.A., Eds.; MIT Publishing: Bremen, Germany, 2001; pp. 17–24. [Google Scholar]
- Hanssen, A.G.; Stöbener, K.; Rausch, G.; Langseth, M.; Keller, H. Optimisation of energy absorption of an A-pillar by metal foam insert. Int. J. Crashworth. 2006, 11, 231–241. [Google Scholar] [CrossRef]
- Viehweger, B.; Sviridov, A. Frontmodule für schienenfahrzeuge aus aluminiumschaumsandwich-fertigungstechnologien zur bauteilherstellung. Forum Forsch. 2007, 20, 69–72. [Google Scholar]
- Martec-era. Available online: https://www.martec-era.net/ (accessed on 27 July 2015).
- Hanssen, A.G.; Girard, Y.; Olovsson, L.; Berstad, T.; Langseth, M. A numerical model for bird strike of aluminium foam-based sandwich panels. Int. J. Impact Eng. 2006, 32, 1127–1144. [Google Scholar] [CrossRef]
- Thoma, K.; Wicklein, M.; Schneider, E. New Protection Concepts for Meteoroid/Debris Shields. In Proceedings of the European Conference on Space Debris, Darmstadt, Germany, 18–20 April 2005; Danesy, D., Ed.; ESA: Darmstadt, Germany; pp. 445–452.
- Ryan, S.; Christiansen, E.; Lear, D. Shielding Against Micrometeoroid and Orbital Debris Impact with Metallic Foams; NASA: Houston, TX, USA, 2011; pp. 267–269. [Google Scholar]
- Alcarbon. Available online: http://www.alcarbon.de/ (accessed on 27 July 2015).
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. ALPORAS aluminum foam: Production process, properties, and applications. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsumoji, T.; Sueki, T.; Takaishi, T. Aerodynamic noise reduction in pantographs by shape-smoothing of the panhead and its support and by use of porous material in surface coverings. Q. Rprt. RTRI 2010, 51, 220–226. [Google Scholar] [CrossRef]
- Mitsumoji, T.; Sueki, T.; Yamazaki, N.; Sato, Y.; Ikeda, M.; Takinami, R.; Gejima, H.; Fukagata, K. Aerodynamic noise reduction of a pantograph panhead by applying a flow control method. In Noise and Vibration Mitigation for Rail Transportation Systems; Nielsen, J.C.O., Anderson, D., Gautier, P.-E., Iida, M., Nelson, J.T., Thompson, D., Tielkes, T., Towers, D.A., de Vos, P., Eds.; Springer: Berlin, Heidelberg, Germany, 2015; Volume 126, pp. 515–522. [Google Scholar]
- Paun, F.; Gasser, S.; Leylekian, L. Design of materials for noise reduction in aircraft engines. Aerosp. Sci. Technol. 2003, 7, 63–72. [Google Scholar] [CrossRef]
- Mott Corporation. Available online: http://www.mottcorp.com/products/ (accessed on 24 July 2015).
- Paserin, V.; Marcuson, S.; Shu, J.; Wilkinson, D.S. The Chemical Vapor Deposition Technique for Inco Nickel Foam Production—Manufacturing Benefits and Potential Applications. In Proceedings of the Cellular Metals and Metal Foaming Technology, Berlin, Germany, 23–25 June 2003; Banhart, J., Fleck, N.A., Eds.; MIT-Verlag: Berlin, Germany; pp. 31–38.
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Bäumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Raval, S. CAMRAS: NASA’s CO2 and Moisture Removal System Ready for Final Tests. Available online: http://www.spacesafetymagazine.com/aerospace-engineering/spacecraft-design/camras-nasas-co2-moisture-removal-system-ready-final-tests/ (accessed on 14 December 2015).
- Montillet, A.; Comiti, J.; Legrand, J. Application of metallic foams in electrochemical reactors of filter-press type Part I: Flow characterization. J. Appl. Electrochem. 1993, 23, 1045–1050. [Google Scholar] [CrossRef]
- Cognet, P.; Berlan, J.; Lacoste, G.; Fabre, P.L.; Jud, J.M. Application of metallic foams in an electrochemical pulsed flow reactor Part II: Oxidation of benzyl alcohol. J. Appl. Electrochem. 1996, 26, 631–637. [Google Scholar] [CrossRef]
- Treviño, P.; Ibanez, J.G.; Vasquez-Medrano, R. Chromium (VI) reduction kinetics by zero-valent aluminum. Int. J. Electrochem. Sci. 2014, 9, 2556–2564. [Google Scholar]
- Langlois, S.; Coeuret, F. Flow-through and flow-by porous electrodes of nickel foam. II. diffusion-convective mass transfer between the electrolyte and the foam. J. Appl. Electrochem. 1989, 19, 51–60. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Moreno, F. Commercial Applications of Metal Foams: Their Properties and Production. Materials 2016, 9, 85. https://doi.org/10.3390/ma9020085
García-Moreno F. Commercial Applications of Metal Foams: Their Properties and Production. Materials. 2016; 9(2):85. https://doi.org/10.3390/ma9020085
Chicago/Turabian StyleGarcía-Moreno, Francisco. 2016. "Commercial Applications of Metal Foams: Their Properties and Production" Materials 9, no. 2: 85. https://doi.org/10.3390/ma9020085