Preparation and Characterization of All-Biomass Soy Protein Isolate-Based Films Enhanced by Epoxy Castor Oil Acid Sodium and Hydroxypropyl Cellulose
Abstract
:1. Introduction
2. Experimental
2.1. Materials
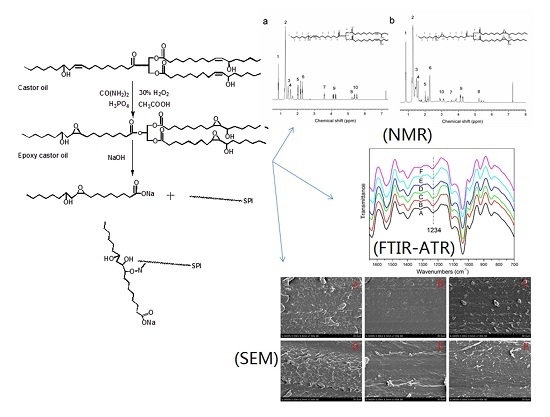
2.2. Synthesis of ECO
2.3. Preparation of SPI films
2.4. 1H Nuclear Magnetic Resonance (NMR)
2.5. Film Characterization
2.5.1. Equilibrium Treatment
2.5.2. Film Thickness
2.5.3. Mechanical Properties
2.5.4. X-ray Diffraction Analysis (XRD)
2.5.5. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5.6. Thermogravimetric Analysis (TGA)
2.5.7. Scanning Electron Microscopy (SEM)
2.5.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of ECO
3.2. ATR-FTIR Spectra of the SPI-Based Films
3.3. Thermal Properties of the SPI-Based Films
3.4. Crystalline Properties of the SPI-Based Films
3.5. Micromorphology of the SPI-Based Films
3.6. Physical and Mechanical Properties of the SPI-Based Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ammala, A.; Bateman, S.; Dean, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Polym. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Zhang, H.; Mittal, G. Biodegradable protein-based films from plant resources: A review. Prog. Sustain. Energy 2010, 29, 203–220. [Google Scholar] [CrossRef]
- Xia, C.; Shi, S.Q.; Cai, L. Vacuum-assisted resin infusion (VARI) and hot pressing for CaCO3 nanoparticle treated kenaf fiber reinforced composites. Compos. B 2015, 78, 138–143. [Google Scholar] [CrossRef]
- Xia, C.; Shi, S.Q.; Cai, L.; Hua, J. Property enhancement of kenaf fiber composites by means of vacuum-assisted resin transfer molding (VARTM). Holzforschung 2015, 69, 307–312. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocolloids 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Kim, K.M.; Weller, C.L.; Hanna, M.A.; Gennadios, A. Heat curing of soy protein films at selected temperatures and pressures. LWT-Food. Sci. Technol. 2002, 35, 140–145. [Google Scholar] [CrossRef]
- Monedero, F.M.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of calcium and sodium caseinates on physical characteristics of soy protein isolate–lipid films. J. Food Eng. 2010, 97, 228–234. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Miiller, K.; Rodriguze, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their ppplicability to cellulose based products: An extensive review. Coatings. 2015, 6, 1. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and quantification of molecular interactions in protein films: A review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Tien, C.; Vachon, C.; Mateescu, M.A.; Lacroix, M. Milk protein coatings prevent oxidative browning of apples and potatoes. J. Food. Sci. 2001, 66, 512–516. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible films and coatings from whey proteins: A review on formulation, and on mechanical and bioactive properties. Crit. Rev. Food Sci. 2012, 52, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Misra, M.; Askeland, P.; Drzal, L.T.; Mohanty, A.K. ‘Green’composites from soy based plastic and pineapple leaf fiber: Fabrication and properties evaluation. Polymer 2005, 46, 2710–2721. [Google Scholar] [CrossRef]
- Tummala, P.; Liu, W.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Influence of plasticizers on thermal and mechanical properties and morphology of soy-based bioplastics. Ind. Eng. Chem. Res. 2006, 45, 7491–7496. [Google Scholar] [CrossRef]
- Wittaya, T. Protein-based edible films: Characteristics and improvement of properties. In Structure and Function of Food Engineering; INTECH: Rijeka, Croatia, 2012. [Google Scholar]
- Zhang, S.; Xia, C.; Dong, Y.; Yan, Y.; Li, J.; Shi, S.Q.; Cai, L. Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Ind. Crops Prod. 2016, 80, 207–213. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by PTGE and PAM. Ind. Crops Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, S.; Shi, S.Q.; Cai, L.; Garcia, A.C.; Rizvi, H.R.; D’Souza, N.A. Property enhancement of soy protein isolate-based films by introducing POSS. Int. J. Biol. Macromol. 2016, 82, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Zheng, H. Effect of transglutaminase on properties of tilapia scale gelatin films incorporated with soy protein isolate. Food. chem. 2015, 169, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, H.; Wei, M.; Huang, J.; Chen, Y. Structure and mechanical properties of cellulose derivatives/soy protein isolate blends. J. Appl. Polym. Sci. 2008, 107, 3267–3274. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Qin, Z.; Chen, H.; Gao, Q.; Li, J. Mechanical and thermal properties of microcrystalline cellulose-reinforced soy protein isolate–gelatin eco-friendly films. RSC Adv. 2015, 5, 56518–56525. [Google Scholar] [CrossRef]
- Friesen, K.; Chang, C.; Nickerson, M. Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: Mechanical, barrier and cross-linking properties. Food Chem. 2015, 172, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, L. Antimicrobial and antioxidant surface modification of cellulose fibers using layer-by-layer deposition of chitosan and lignosulfonates. Carbohydr. Polym. 2015, 124, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Dusek, K.; Duskova-Smrckova, M.; Zlatanic, A.; Petrovic, Z. Formation of polyurethane networks from polyols based on vegetable oils. Polym. Mat. Sci. Eng. 2002, 223, 381–382. [Google Scholar]
- Petrović, Z.S.; Zlatanić, A.; Lava, C.C.; Sinadinović-Fišer, S. Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids—Kinetics and side reactions. Eur. J. Lipid Sci. Technol. 2002, 104, 293–299. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Benaniba, M.T.; Belhaneche-Bensemra, N.; Gelbard, G. Stabilization of PVC by epoxidized sunflower oil in the presence of zinc and calcium stearates. Polym. Degrad. Stab. 2003, 82, 245–249. [Google Scholar] [CrossRef]
- Tu, Y.C.; Suppes, G.J.; Hsieh, F.H. Thermal and mechanical behavior of flexible polyurethane-molded plastic films and water-blown foams with epoxidized soybean oil. J. Appl. Polym. Sci. 2009, 111, 1311–1317. [Google Scholar] [CrossRef]
- Ahmad, S.; Pk, N.; Riaz, U. Effect of microwave processing on the spectral, mechanical, thermal, and morphological characteristics of sustainable resource based castor oil Epoxy/PVA blends. Adv. Polym. Technol. 2011, 30, 96–109. [Google Scholar] [CrossRef]
- Xia, C.; Wang, L.; Dong, Y.; Zhang, S.; Shi, S.Q.; Cai, L.; Li, J. Soy protein isolate-based films cross-linked by epoxidized soybean oil. RSC Adv. 2015, 5, 82765–82771. [Google Scholar] [CrossRef]
- Xu, F.J.; Zhang, W.; Zhang, S.F.; Li, L.; Li, J.Z.; Zhang, Y. Preparation and characterization of poly (vinyl alcohol) and 1, 2, 3-propanetriol diglycidyl ether incorporated soy protein isolate-based films. J. Appl. Polym. Sci. 2015, 132, 42578–42586. [Google Scholar] [CrossRef]
- Takahashi, T.; Hirayama, K.i.; Teramoto, N.; Shibata, M.; Takahashi, T.; Hirayama, K.; Teramoto, N. Biocomposites composed of epoxidized soybean oil cured with terpene-based acid anhydride and cellulose fibers. J. Appl. Polym. Sci. 2008, 108, 1596–1602. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Bernardi, L.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Development of environmentally friendly composite matrices from epoxidized cottonseed oil. Eur. Polym. 2015, 63, 1–10. [Google Scholar] [CrossRef]
- Luo, Z.; Shi, Y.; Zhao, D.; He, M. Synthesis of epoxidatied castor oil and its effect on the properties of waterborne polyurethane. Procedia Eng. 2011, 18, 37–42. [Google Scholar] [CrossRef]
- Jia, P.Y.; Bo, C.Y.; Zhang, L.Q.; Hu, L.H.; Zhang, M.; Zhou, Y.H. Synthesis of castor oil based plasticizers containing flame retarded group and their application in poly (vinyl chloride) as secondary plasticizer. J. Ind. Eng. Chem. 2015, 28, 217–224. [Google Scholar] [CrossRef]
- Kim, N.; Li, Y.; Sun, X.S. Epoxidation of Camelina sativa oil and peel adhesion properties. Ind. Crops Prod. 2015, 64, 1–8. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocolloids 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Lei, H.; Du, G.; Wu, Z.; Xi, X.; Dong, Z. Cross-linked soy-based wood adhesives for plywood. Int. J. Adhes. Adhes. 2014, 50, 199–203. [Google Scholar] [CrossRef]
- Ogawa, A.; Nakayama, S.; Uehara, M.; Mori, Y.; Takahashi, M.; Aiba, T.; Kurosaki, Y. Pharmaceutical properties of a low-substituted hydroxypropyl cellulose (L-HPC) hydrogel as a novel external dressing. Int. J. Pharm. 2014, 477, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the domain structure of the 7S and 11S globulins from soy proteins by XRD and FTIR. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Tseng, Y.C.; Goldman, D.; Bogner, R.H. Hydrogen bonding with adsorbent during storage governs drug dissolution from solid-dispersion granules. Pharm. Res. 2002, 19, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Weng, L.; Zhang, L. Moephology and properties of soy protein isolate thermoplastics reinforced with chitin whiskers. Biomacromolecules 2004, 5, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.C.; Lee, S.K.; Lee, K.S.; Choi, K.Y. Chain length effect of alkenyl succinic anhydride on thermal and mechanical properties of the cured epoxy resins. Angew. Makromol. Chem. 1991, 185, 129–136. [Google Scholar] [CrossRef]
- Choi, M.H.; Byun, H.Y.; Chung, I.J. The effect of chain length of flexible diacid on morphology and mechanical property of modified phenolic resin. Polymer 2002, 43, 4437–4444. [Google Scholar] [CrossRef]







| Sample | SPI (g) | Glycerol (g) | Water (g) | HPC (g) | ECOS (g) | ASA (g) |
|---|---|---|---|---|---|---|
| A | 5 | 2.5 | 95 | – | – | – |
| B | 5 | 2.5 | 95 | 0.05 | – | – |
| C | 5 | 2.5 | 95 | 0.1 | – | – |
| D | 5 | 2.5 | 95 | 0.25 | – | – |
| E | 5 | 2.5 | 95 | 0.1 | 0.5 | – |
| F | 5 | 2.5 | 95 | 0.1 | 0.5 | 0.005 |
| Films | Ti1 (°C) | Tmax1 (°C) | Ti2 (°C) | Tmax2 (°C) |
|---|---|---|---|---|
| A 1 | 131.20 | 159.44 | 270.73 | 299.92 |
| B 2 | 132.12 | 162.56 | 272.73 | 302.88 |
| C 3 | 130.38 | 161.50 | 272.20 | 301.07 |
| D 4 | 126.80 | 154.96 | 272.86 | 302.12 |
| E 5 | 137.52 | 171.11 | 284.56 | 299.36 |
| F 6 | 137.54 | 167.53 | 283.54 | 297.45 |
| Films | TS (MPa) | EB (%) | Thickness (mm) |
|---|---|---|---|
| Average (SD) | Average (SD) | Average (SD) | |
| A 1 | 2.84 (0.140) a | 220.4 (4.0) a | 0.239 (0.006) a |
| B 2 | 3.17 (0.132) b | 221.8 (22.7) a | 0.231 (0.021) a |
| C 3 | 3.63 (0.133) c | 227.9 (8.8) ab | 0.233 (0.017) a |
| D 4 | 3.38 (0.175) bd | 271.8 (18.3) c | 0.263 (0.009) b |
| E 5 | 3.75 (0.058) ce | 257.3 (25.5) bc | 0.207 (0.008) c |
| F 6 | 4.04 (0.171) f | 270.4 (11.3) c | 0.244 (0.015) d |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, J.; Zhang, S.; Shi, J. Preparation and Characterization of All-Biomass Soy Protein Isolate-Based Films Enhanced by Epoxy Castor Oil Acid Sodium and Hydroxypropyl Cellulose. Materials 2016, 9, 193. https://doi.org/10.3390/ma9030193
Wang L, Li J, Zhang S, Shi J. Preparation and Characterization of All-Biomass Soy Protein Isolate-Based Films Enhanced by Epoxy Castor Oil Acid Sodium and Hydroxypropyl Cellulose. Materials. 2016; 9(3):193. https://doi.org/10.3390/ma9030193
Chicago/Turabian StyleWang, La, Jianzhang Li, Shifeng Zhang, and Junyou Shi. 2016. "Preparation and Characterization of All-Biomass Soy Protein Isolate-Based Films Enhanced by Epoxy Castor Oil Acid Sodium and Hydroxypropyl Cellulose" Materials 9, no. 3: 193. https://doi.org/10.3390/ma9030193