3.2. Isothermal Section based on Diffusion Couples and Key Sample Analysis
In addition to the diffusion couples 32 key alloys were studied in order to construct the isothermal section at 673 K. The BSE images of selected key alloys are shown in
Figure 13. The WDS analysis of all the key samples was performed, the samples were grouped based on the phase analysis and are discussed accordingly. The occurrence of twelve ternary compounds (τ
1–τ
12) in the Mg-Ni-Y system was confirmed. Among these only two, τ
3 and τ
4 were reported in the literature [
12,
13,
14,
15]. Based on the current analysis, approximate composition of all the compounds was obtained. The composition and homogeneity range of these ternary compounds are listed in
Table 1. The ternary solubility of the binary compounds was also determined. The maximum Ni solubility in MgY(γ), Mg
2Y(δ) and Mg
24Y
5(ε) was found to be ~1.0 at% Ni. Among the Ni-Y compounds, Ni
17Y
2 and NiY dissolved about 3.5 and 1.3 at% Mg. The solubility of Mg in Ni
3Y, Ni
4Y and NiY
3 is negligible (~0.5 ± 1 at% Mg). Both, Mg
2Ni and MgNi
2 were found to dissolve about 4.0 at% Y.
Figure 13.
BSE image of (a) sample 1 (19.5/29.5/51.0 Mg/Ni/Y at%); (b) sample 9 (25.0/30.3/44.7 Mg/Ni/Y at%); (c) sample 11 (26.0/38.2/35.8 Mg/Ni/Y at%); (d) Sample 18 (28.7/69.1/2.2 Mg/Ni/Y at%); (e) sample 25 (78.2/15.5/6.3 Mg/Ni/Y at%); (f) sample 30 (54.0/15.9/30.1 Mg/Ni/Y at%).
The presence of the ternary compound τ
1 and the phase relationship in the Y-rich portion of the phase diagram were confirmed by the analysis of eight key samples 1–8. The actual composition of the alloys are shown in
Figure 2. In all these alloys, τ
1 is positively identified. The WDS analysis listed in
Table 2 shows a range of composition (~15–20 at% Mg, ~16–20 at% Ni) of τ
1 suggesting solid solubility. Variation in all the three elements indicates random substitution of atoms. Therefore, a relatively round region has been assigned to demonstrate the solubility range of τ
1. According to the WDS analysis of τ
1 containing alloys (1–8), five three-phase regions were also established: τ
1 + NiY + Ni
2Y
3, τ
1 + NiY
3 + Ni
2Y
3, τ
1 + NiY + τ
5, τ
1 + MgY + τ
5 and τ
1 + NiY
3 + hcp-Y.
Table 1.
Ternary intermetallic compounds in the Mg-Ni-Y system.
| Phase | Crystal Structure | Lattice Parameter (nm) | Homogeneity Range | Ref. |
|---|
| a | c |
|---|
| τ1-MgNiY4 | Gd4RhIn | 1.3666 | | 15–20 at% Mg | This work |
| 16–20 at% Ni |
| τ2-MgNi2Y2 | Mo2FeB2 | 0.7395(9) | 0.3736(3) | | This work |
| τ3-MgNi4Y | MgCu4Sn | 0.71853 | | 11–23 at% Mg | This work |
| ~66.6 at% Ni |
| τ4-Mg2Ni9Y | Mg2Ni9La | 0.48666(5) | 2.37733(5) | | [12] |
| τ5-Mg29Ni20Y42 | Unknown | | | | This work |
| τ6-MgNiY | Unknown | | | | This work |
| τ7-Mg8Ni5Y5 | Unknown | | | | This work |
| τ8-Mg2NiY | Unknown | | | | This work |
| τ9-Mg57Ni18Y25 | Unknown | | | | This work |
| τ10-Mg6NiY | Unknown | | | | This work |
| τ11-Mg9NiY | Unknown | | | 85.6–89.0 at% Mg | This work |
| at%Ni/at%Y 1 |
| τ12-Mg15NiY | Unknown | | | 81.6–85.0 at% Mg | This work |
| at%Ni/at%Y 1 |
Table 2.
Wave dispersive X-ray spectrometer (WDS) data of the samples 1–8 annealed at 673 K.
| Actual Composition | Identified Phases |
|---|
| No. | at% | Name | Composition by WDS |
|---|
| Mg | Ni | Y | Mg | Ni | Y |
|---|
| 1 | 19.5 | 29.5 | 51.0 | τ1 | 20.9 | 16.6 | 62.5 |
| τ5 | 33.6 | 21.9 | 44.5 |
| NiY | 0.9 | 48.9 | 50.2 |
| 2 | 18.5 | 22.4 | 59.1 | τ1 | 20.1 | 17.3 | 62.6 |
| τ5 | 31.7 | 21.6 | 46.7 |
| NiY | 0.4 | 48.7 | 50.9 |
| 3 | 13.1 | 26.7 | 60.2 | τ1 | 16.4 | 20.3 | 63.3 |
| Eutectic | 12.96 | 29.1 | 58.0 |
| 4 | 4.8 | 28.3 | 66.9 | τ1 | 16.4 | 21.3 | 62.3 |
| NiY3 | 0.9 | 24.9 | 74.2 |
| NiY | 0.4 | 48.8 | 50.8 |
| 5 | 3.9 | 23.6 | 72.5 | τ1 | 14.7 | 22.1 | 63.2 |
| NiY3 | 0.7 | 25.5 | 73.8 |
| 6 | 5.9 | 20.9 | 73.2 | τ1 | 16.2 | 18.6 | 65.2 |
| NiY3 | 1.2 | 24.3 | 74.5 |
| 7 | 8.6 | 16.5 | 74.9 | τ1 | 17.2 | 16.5 | 66.2 |
| NiY3 | 0.9 | 24.4 | 74.7 |
| Y-hcp | 0.7 | 0.8 | 98.5 |
| 8 | 37.0 | 15.0 | 48.0 | MgY(γ) | 51.5 | 1.6 | 46.9 |
| τ1 | 26.3 | 15.6 | 58.1 |
| τ5 | 32.8 | 21.4 | 45.8 |
Hara
et al. [
7] in their hydrogen storage work on the Mg-Ni-Y system, reported an unknown phase of composition MgNiY
3. They reported that this compound plays a role as a catalyst for the adsorption of hydrogen. However, they could not identify the crystal structure of this compound. The composition of this compound (MgNiY
3) is very close to the single phase region of τ
1. Hence, it is likely that Hara
et al. [
7] were actually detected τ
1 (MgNiY
4).
The crystallographic information of τ
1 was determined in this work. This was done by comparing XRD patterns of several τ
1 containing alloys (KS# 1–8) with similar structure type compounds from Pearson crystal structure database [
27]. It was found that the XRD pattern of Gd
4RhIn was very similar to that of τ
1. Tappe
et al. [
28] reported several rare earth containing compounds with this prototype. Therefore, the crystallographic data of Gd
4RhIn was taken as the starting value for the Rietveld structural refinement of τ
1 in the present work. Using this strategy the lattice parameter of τ
1 was determined as:
a = 1.3666 nm. The refined crystal structure data and atomic positions of τ
1 are listed in
Table 3 and
Table 4. Also, the unit cell is shown in
Figure 14a. The XRD pattern of sample 2, shown in
Figure 15, positively identifies τ
1 and NiY. The SEM analysis of this alloy as listed in
Table 2 showed three phases, τ
1 + NiY + τ
5. Therefore it can be said that the unknown peaks in the XRD pattern belong to τ
5. Since the crystal structure of τ
5 is unknown these peaks are labeled by a question mark (?). The statistically expected values Re (12.6), weighted summation of residuals of the least squared fit R
wp (24.9) and goodness of fit s (3.9) were used to judge the degree of refinement in the Rietveld analysis. Due to the unknown peaks of τ
5, a better statistical fit was not possible.
Table 3.
The crystal structure data of τ1 and τ2.
| Compound | MgNiY4 (τ1) | MgNi2Y2 (τ2) |
|---|
| Structure | Cubic | Tetragonal |
| Prototype | Gd4RhIn | Mo2FeB2 |
| Space group | (216) | P4/mbm (127) |
| Lattice parameter (nm) | a | a | c |
| 1.3666(0) | 0.7395(9) | 0.3736(3) |
| Angles | α | β | γ | α | β | γ |
| 90° | 90° | 90° | 90° | 90° | 90° |
| Atoms in unit cell | 96 | 10 |
Table 4.
Atoms position in the unit cell of τ1 and τ2.
| Atom | Wyckoff Position | x | y | z |
|---|
| MgNiY4 (τ1) |
| Mg | 16e | 0.080 | 0.080 | 0.080 |
| Ni | 16e | 0.640 | 0.640 | 0.640 |
| Y1 | 24g | 0.560 | 0.250 | 0.250 |
| Y2 | 24f | 0.310 | 0 | 0 |
| Y3 | 16e | 0.153 | 0.153 | 0.153 |
| MgNi2Y2 (τ2) |
| Mg | 2a (12) | 0 | 0 | 0 |
| Ni | 4g (9) | 0.6216 | 0.1216 | 0 |
| Y | 4h (17) | 0.1716 | 0.6716 | 0.5000 |
Figure 14.
Unit cell for (a) τ1 and (b) τ2.
Figure 15.
X-ray diffraction (XRD) pattern for sample 2 (18.5/22.4/59.1 Mg/Ni/Y at%).
The ternary compound, τ
2 was not observed in any of the diffusion couples. Therefore more effort was dedicated towards confirming its presence. Five key alloys (9–13) were prepared near this compound. τ
2 was found to exist in all of them. According to the WDS analysis in
Table 5, the composition of this compound is 20/40/40 Mg/Ni/Y at%. Based on this, the MgNi
2Y
2 formula was assigned to it. These key alloys also demonstrated the occurrence of three 3-phase regions, τ
2 + τ
3 + NiY, τ
2 + τ
5 + NiY and τ
2 + τ
3 + τ
6.
The crystallographic information of τ
2 was determined in the present work in a similar approach to that of τ
1 by comparing the XRD patterns of several alloys containing this phase with similar structure type compounds from the Pearson crystal structure database [
27]. It was found that τ
2 has Mo
2FeB
2 prototype and the lattice parameters were determined as
a = 0.7395(9) nm and
c = 0.3736(3) nm. The crystal structure data and atoms position within the unit cell are listed in
Table 3 and
Table 4. Also, the unit cell of τ
2 is shown in
Figure 14b.
Table 5.
WDS data of the samples 19–26 annealed at 673 K.
| Actual Composition | Identified Phases |
|---|
| No. | at% | Name | Composition by WDS |
|---|
| Mg | Ni | Y | Mg | Ni | Y |
|---|
| 9 | 25.0 | 30.3 | 44.7 | τ2 | 20.9 | 37.9 | 41.2 |
| τ5 | 35.0 | 21.5 | 43.5 |
| NiY | 0.3 | 49.2 | 50.5 |
| 10 | 28.0 | 36.8 | 35.2 | τ2 | 22.4 | 39.3 | 38.3 |
| τ3 | 16.3 | 64.2 | 19.5 |
| τ6 | 35.9 | 32.7 | 31.4 |
| 11 | 26.0 | 38.2 | 35.8 | τ2 | 21.4 | 39.2 | 39.4 |
| τ3 | 16.4 | 64.0 | 19.6 |
| τ6 | 35.6 | 32.3 | 32.1 |
| 12 | 20.0 | 50.8 | 29.2 | τ2 | 19.7 | 40.5 | 39.8 |
| τ3 | 11.8 | 66.5 | 21.7 |
| τ6 | 32.2 | 34.3 | 33.5 |
| 13 | 8.4 | 58.6 | 33.0 | NiY | 1.3 | 49.6 | 49.1 |
| τ2 | 21.2 | 39.3 | 39.5 |
| τ3 | 18.0 | 66.5 | 15.5 |
XRD patterns of key alloys 10, 11 and 12 are shown in
Figure 16a–c. τ
2 was positively identified in all of them. The unknown phase in these XRD patterns belongs to τ
6. Another ternary compound, τ
3 was also positively identified in these XRD patterns. This compound was first reported by Kadir
et al. [
14]. They reported SnMgCu
4 prototype with lattice parameter of 0.71853 nm. This compound was found in several alloys (10–13, 16, 19–25) in the current work. According to the WDS analysis in
Table 5 and
Table 6, τ
3 has solubility from ~11 to 23 at% Mg with constant 66.67 at% Ni. The variation of lattice parameter of τ
3 was also observed in the Rietveld analysis performed on the XRD patterns of four key alloys (5, 9, 10 and 11). The change in lattice parameter, “
a” of τ
3 with Mg concentration is presented in
Figure 17. The atomic radius of Mg (145 pm) is less than that of Y (212 pm). According to Vegard’s law, the decrease of size of any atom or ion in a crystal leads directly to the proportional decrease of lattice constants. Hence the lattice parameter “
a” decreases with the increase of Mg concentration. It reflects substitutional solid solution for τ
3 where Mg replaces Y atoms.
τ
4 (Mg
2Ni
9Y) was identified in sample 17 (11.0/78.9/10.1 Mg/Ni/Y at%) and sample 18 (28.7/69.1/2.2 Mg/Ni/Y at%). Kadir
et al. Reference [
12] reported a hexagonal structure for τ
4, which is iso-structure of LaMg
2Ni
9. Two different plate-like structures can be seen in the BSE image of sample 18 in
Figure 13d. These were identified as MgNi
2 and τ
4.
The intermetallic compound, τ
5 was found in samples 1, 2, 8, 9 and 27–29. Based on the WDS analysis of these alloys in
Table 5 and
Table 7, the approximate composition of τ
5 was determined as 33.8/21.5/44.7 Mg/Ni/Y at%. The BSE image of sample 9 (25.0/30.3/44.7 Mg/Ni/Y at%) in
Figure 13b clearly shows τ
5 which is in equilibrium with τ
2 and NiY. The crystal structure of τ
5 is not known. Determining the crystal structure of τ
5 in the same approach as for τ
1 and τ
2 was not successful because a crystallographic prototype has not been found yet.
Figure 16.
XRD patterns for (a) Sample 10 (20.0/50.8/29.2 Mg/Ni/Y at%); (b) Sample 11 (26.0/38.2/35.8 Mg/Ni/Y at%); (c) sample 12 (28.0/36.8/35.2 Mg/Ni/Y at%).
Figure 17.
Variation of lattice parameter of τ3.
Table 6.
WDS data of the samples 14–26 annealed at 673 K.
| Actual Composition | Identified Phases |
|---|
| No. | at% | Name | Composition by WDS |
|---|
| Mg | Ni | Y | Mg | Ni | Y |
|---|
| 14 | 1.5 | 63.4 | 35.1 | Ni2Y | 0.7 | 63.1 | 36.2 |
| NiY | 0.5 | 46.7 | 52.8 |
| 15 | 3.3 | 80.5 | 16.2 | Ni5Y | 0.7 | 82.9 | 16.4 |
| τ4 | 15.5 | 74.7 | 9.8 |
| 16 | 3.7 | 77.3 | 19.0 | Ni5Y | 0.4 | 82.9 | 16.7 |
| τ3 | 9.8 | 66.9 | 23.3 |
| Ni4Y | 1.3 | 78.8 | 19.9 |
| 17 | 11.0 | 78.9 | 10.1 | Ni17Y2 | 3.5 | 84.9 | 11.6 |
| τ4 | 17.6 | 75.6 | 6.8 |
| 18 | 28.7 | 69.1 | 2.2 | MgNi2 | 31.3 | 66.8 | 1.8 |
| τ4 | 16.5 | 75.4 | 8.1 |
| 19 | 36.2 | 59.9 | 3.9 | Mg2Ni | 67.2 | 32.5 | 0.3 |
| τ3 | 25.9 | 64.9 | 9.2 |
| MgNi2 | 31.4 | 64.3 | 4.3 |
| 20 | 27.2 | 59.9 | 12.9 | Mg2Ni | 67.2 | 32.5 | 0.3 |
| τ3 | 22.7 | 64.9 | 12.3 |
| 21 | 24.5 | 52.4 | 23.1 | τ3 | 15.1 | 64.7 | 20.2 |
| τ7 | 45.9 | 27.1 | 27.0 |
| τ8 | 51.2 | 24.5 | 24.3 |
| 22 * | 37.2 | 36.3 | 26.5 | τ3 | 15.6 | 65.1 | 19.3 |
| τ6 | 34.6 | 31.7 | 33.7 |
| τ8 | 51.2 | 24.4 | 24.4 |
| τ10 | 74.1 | 13.0 | 12.9 |
| τ11 | 82.5 | 8.6 | 8.9 |
| 23 * | 54.1 | 24.0 | 21.9 | τ3 | 17.8 | 64.1 | 18.1 |
| τ8 | 52.3 | 24.3 | 23.4 |
| τ10 | 74.5 | 12.8 | 12.7 |
| τ11 | 84.5 | 7.6 | 7.9 |
| 24 | 55.5 | 36.6 | 7.9 | τ12 | 85.1 | 6.7 | 8.2 |
| Mg2Ni | 65.3 | 33.8 | 0.9 |
| τ3 | 17.4 | 66.8 | 15.8 |
| 25 | 78.2 | 15.5 | 6.3 | τ12 | 85.3 | 6.3 | 8.4 |
| Mg2Ni | 67.5 | 30.7 | 1.8 |
| τ3 | 18.8 | 64.5 | 16.5 |
| 26 | 84.9 | 12.9 | 2.2 | hcp-Mg | 99.4 | 0.5 | 0.1 |
| τ12 | 92.0 | 4.1 | 3.9 |
| Mg2Ni | 67.9 | 30.2 | 1.9 |
The BSE images of sample 22 (37.2/36.3/26.5 Mg/Ni/Y at%) and sample 29 (51.4/12.3/36.3 Mg/Ni/Y at%) in
Figure 18a,b, show the presence of τ
6. This compound was also found in samples 10–12 and 29. In order to obtain equilibrium, these alloys were annealed for six weeks instead of four. Still complete equilibrium could not be obtained. This is probably due to the peritectic decomposition of some of the compounds. The BSE image of sample 22 in
Figure 18a shows several phases; τ
3, τ
6, τ
8, τ
10, and τ
11. Based on the analysis of samples 22 and 23 and the diffusion couples, it can be concluded that τ
3 is in equilibrium with all the compounds in the vicinity of the Mg-NiY line.
Although not in complete equilibrium, a three-phase relation among Mg
2Y(δ), τ
5 and τ
6 can be identified in sample 29 (51.4/12.3/36.3 Mg/Ni/Y at%) in
Figure 18b. It is observed that τ
2 (white-square shape) always remains within τ
6 and will probably transfer to τ
6 after a much longer annealing time. This indicates a three-phase region: Mg
2Y(δ) + τ
5 + τ
6. The colors of τ
5 and τ
6 are very close because of their similar composition. This makes it difficult to recognize these two phases in the BSE image.
The ternary compounds τ
7 and τ
8 were found in sample 21 (24.6/52.4/23.0 Mg/Ni/Y at%). τ
8 was also identified in samples 22 and 23. The WDS analysis of these alloys is summarized in
Table 6. Their presence was also confirmed in three diffusion couples (DC{SS} 2, 3 and DC{SL} 2) as shown earlier in
Figure 5,
Figure 7 and
Figure 10, respectively.
Table 7.
WDS data of the samples 27–32 annealed at 673 K.
| Actual Composition | Identified Phases |
|---|
| No. | at% | Name | Composition by WDS |
|---|
| Mg | Ni | Y | Mg | Ni | Y |
|---|
| 27 | 38.5 | 14.7 | 46.8 | MgY(γ) | 49.6 | 1.1 | 49.3 |
| τ5 | 34.1 | 21.5 | 44.4 |
| Mg2Y(δ) | - | - | - |
| 28 | 49.9 | 8.0 | 42.1 | Mg2Y(δ) | 68.6 | 0.4 | 31.0 |
| MgY(γ) | 53.4 | 0.8 | 45.8 |
| τ5 | 34.1 | 21.1 | 44.8 |
| 29 * | 51.4 | 12.3 | 36.3 | τ2 | 21.4 | 37.5 | 41.1 |
| τ5 | 35.7 | 21.6 | 42.7 |
| τ6 | 36.2 | 30.8 | 33.0 |
| Mg2Y(δ) | 71.7 | 0.8 | 27.5 |
| 30 | 54.0 | 15.9 | 30.1 | τ6 | 35.1 | 32.4 | 32.5 |
| τ9 | 56.7 | 16.5 | 26.8 |
| Mg2Y(δ) | 69.1 | 1.5 | 29.4 |
| 31 | 69.1 | 9.4 | 21.5 | τ9 | 57.5 | 16.4 | 26.1 |
| τ10 | 67.0 | 14.1 | 18.9 |
| Mg2Y(δ) | 72.6 | 1.4 | 26.0 |
| 32 | 72.0 | 6.3 | 21.7 | τ10 | 67.1 | 12.3 | 20.6 |
| Mg2Y(δ) | 73.5 | 1.1 | 25.4 |
Figure 18.
BSE image of (a) sample 22 (37.2/36.3/26.5 Mg/Ni/Y at%); (b) Sample 29 (51.4/12.3/36.3 Mg/Ni/Y at%).
The BSE image of sample 30 (54.0/15.9/30.1 Mg/Ni/Y at%) in
Figure 13f shows a three-phase equilibrium among Mg
2Y(δ), τ
6 and τ
9. The white flakes and the grey matrix in the microstructure were identified as τ
6 and τ
9, respectively. Mg
2Y(δ) was found as fine precipitates in the grey matrix. According to the WDS analysis of sample 30 in
Table 7, the composition of the ternary compound τ
9 is 56.7/16.5/26.8 Mg/Ni/Y at%. This compound was also observed in the solid-solid diffusion couple 1 in
Figure 3 with slightly different composition 59.1/16.5/24.3 Mg/Ni/Y at%. The WDS analysis of sample 31 (69.1/9.4/21.5 Mg/Ni/Y at%) in
Table 7, identified this compound, τ
9, as 57.5/16.4/26.1 Mg/Ni/Y at%, which is closer to the diffusion couple analysis. Therefore, it was decided to use the composition obtained by the diffusion couple analysis since it is generally more accurate.
The WDS analysis of sample 31 (69.1/9.4/21.5 Mg/Ni/Y at%) and sample 32 (72.0/6.3/21.7 Mg/Ni/Y at%) in
Table 7, showed a three-phase, Mg
2Y(δ) + τ
9 + τ
10, and a two-phase, Mg
2Y(δ) + τ
10 equilibria. Both of these alloys confirmed the existence of the ternary compound τ
10 with an average composition 66.4/12.7/20.9 Mg/Ni/Y at%. However, the solid-solid diffusion couple 2 shown in
Figure 5 revealed the composition as 74.7/12.9/12.4 Mg/Ni/Y at%. The compositional variation is probably due to a linear solubility of τ
10 from ~66.4 at% (sample 31 and 32) to 74.7 at% Mg at constant Ni of ~12.7 at%. The diffusion layer for τ
10 in the solid-solid diffusion couple 2, is only 6 μm which was not wide enough for detecting the solubility. Therefore, the solubility of τ
10 could not be confirmed.
Two ternary compounds τ
11 and τ
12 in the Mg-rich corner of the Mg-Ni-Y phase diagram were identified in this work. τ
11 was found in key samples 22 (37.2/36.3/26.5 Mg/Ni/Y at%) and 23 (53.0/25.1/21.9 Mg/Ni/Y at%). Also, solid-solid diffusion couples 2 and 3 in
Figure 5 and
Figure 7, showed the presence of τ
11. It was found that τ
11 has a solid solubility, which extends from ~82 to 85 at% Mg. τ
12 was found in all three solid-solid diffusion couples as well as in key samples 24 (55.5/36.6/7.9 Mg/Ni/Y at%), 25 (78.2/15.5/6.3 Mg/Ni/Y at%) and 26 (84.9/12.9/2.2 Mg/Ni/Y at%). According to the WDS analysis, τ
12 has a solubility range, which extends from ~85 to 89 at% Mg.
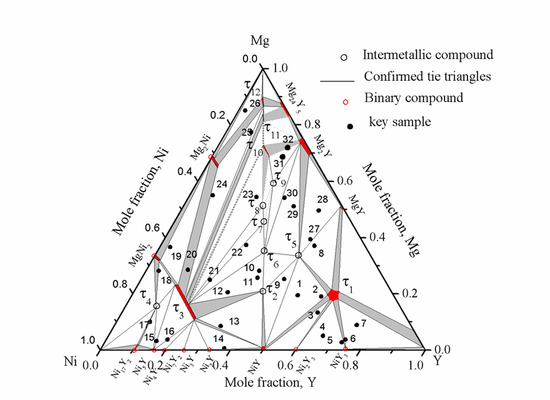
Based on the current results, an isothermal section of the Mg-Ni-Y system at 673 K was constructed as shown in
Figure 19. The dotted lines in the isothermal section show the tentative phase relations, as these could not be confirmed experimentally due to very sluggish kinetics.
Figure 19.
Isothermal section of the Mg-Ni-Y system at 673 K for the whole composition.