1. Introduction
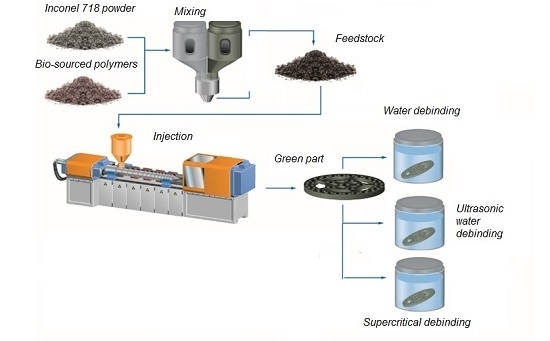
Metal injection molding (MIM) is a process to produce on mass small parts of less than 50 g with complex geometry from varied materials such as stainless steel, superalloys, carbides or ceramics. The MIM process provides good dimensional accuracy (tolerances < 5 microns), a good surface finish (
Ra < 1.5 microns) and high mechanical strength similar to those of the powder material [
1,
2]. The MIM is a process that involves many industrial sectors such as automotive, aerospace, information technology or medical. This process is based on the injection of a fluid material composed of powder of the desired material for the final part, and of a binder of several polymers. The piece is then subjected to debinding to remove the binder and then the piece is sintered to obtain a dense part [
2].
The binder plays the most important role in the MIM process. It has to be able to support an important load rate of powder, typically 60%, and to carry the powder in the mold. Enneti, Quinard and Tam [
2,
3,
4] showed that the binder must give strength and cohesion to the molded part and be easily removed from the molded part, while being recyclable, environmentally friendly and economical. These authors also explained the importance of a low viscosity, good adhesion to the powder, no chemical reaction with the powder which can contaminate the powder or scissor the polymeric chains, and a low coefficient of thermal expansion for binders. The binders are generally composed of three components [
2]. One provides the necessary fluidity (polyethylene glycol (PEG) or waxes are generally used). Another one serves to provide strength of the injected piece, for which polypropylene or polyethylene is commonly employed. The last one is a surfactant which prevents the possible aggregation of the powder particles. Stearic acid is often used.
The different polymers of the binders used in this study are choose due to their environmentally friendly behavior in the MIM process. First the PEG permits the realization a water debinding due to its solubility in water [
2,
5]. This is environmentally important because the solvent debinding is generally realized using hazardous chemical solvent. Moreover the water and the PEG can be separated and then the water recycled. The PEG is a biocompatible thermoplastic semi-crystalline homopolymer which his characteristics depend on the molecular mass. It contains terminal hydroxyl groups which provides water-solubility for molecular masses ranging from 400 to 40,000 g·mol
−1 [
5,
6,
7]. PEG is not soluble in hydrocarbons but is soluble in acetone and alcohol [
5]. Accordingly, a PEG with a molecular weight of 20,000 g·mol
−1 was chosen as it ensures solubility in water while keeping a sufficient binder viscosity.
The choice of the primary binder polymer is driven by its affinity with the PEG. Moreover, to reduce its environmental impact, bio-sourced polymer was choosen because the primary binder has to be removed by thermal debinding to make the shape of the piece as long as possible. In addition, the influence of the binder on both homogeneity and rheological properties of the feedstock has been established [
8]. Taking into account all these aspects, polyhydroxyalcanoates (PHA) was choose because it is well known to be miscible with the PEG [
5]. Moreover, PHA is one of the most common bio sourced polymers and grades are available for mold injection application [
9]. The PHA is a polyester obtained directly from the bacterial metabolism. More than 250 species of bacteria synthesized PHAs granules size from 0.2 to 0.5 µm as carbon and energy storage materials under condition of limiting nutrients and in the presence of excess carbon source. PHA is a biodegradable and biocompatible polymer with good physical, mechanical and thermal properties [
5,
9].
In this study, a micro powder of Inconel 718 was investigated for an application in the MIM process. A micro powder was chosen because it is well adapted for µMIM experiments, including injection, debinding and sintering stages [
3,
10]. The use of Inconel for MIM process has been studied by Özgün [
11] and a formulation was developed. Inconel superalloys are used in aviation, aerospace and nuclear power due to their high resistance to corrosion and oxidation but also for their excellent mechanical strength at high temperature [
11]. Currently, the most popular superalloys used for the above applications are Inconel 718 and 625. Here, an Inconel 718 powder was chosen as the final material of the piece. The binders developed by Özgün [
11] were composed of polypropylene (PP), carnauba wax, paraffin wax and stearic acid.
The process to remove the PEG using the CO
2 supercritical as solvent was studied. This process heated and pressurised the CO
2 below his critical point (304.1 K and 7.38 MPa). The supercritical CO
2 has the behavior of a gas: this facilitates the penetration of the CO
2 inside the samples, and of a liquid: this increases the solubility of the PEG in the CO
2. The debinding method using the CO
2 supercritical as solvent was first reported by Chartier
et al. in 1995 [
12] but the tests were performed on feedstocks made of different waxes and ceramics powders. Shimizu
et al. [
13] also used this method of debinding with feedstock made of paraffin wax. Federzoni
et al. [
14] were the first to use the CO
2 supercritical as solvent debinding method to remove the PEG. Federzoni
et al. [
14] showed that the PEG can be removed entirely by using 65 °C, 300 bar and 7 h as conditions of the process.
The goal of the present study is to develop an environmentally friendly binder formulation adapted to the use of a micro powder of Inconel 718 with the use of bio sourced polymers as binder and the CO2 supercritical as solvent debinding method.
2. Materials and Methods
Two grades of PHA are used, namely PHA and PHBV provided by NaturePlast and adapted to the injection process. The powder used is an Inconel 718 atomized by argon provide by Sandvik Osprey (Neath, UK). The powder was characterized by a granulometer laser LA-950 V2 provided by Horiba (Kyoto, Japan), the tests were realized in water.
The polymer blends are made in a twin screw mixer provided by Brabender (Duisburg, Germany) which has a volume capacity of 50 cm
3, a speed of 50 rpm and at a temperature of 180 °C. The temperature of the test is determined between the higher melting temperature of the polymer and the lowest temperature of degradation of the polymers. According to this requirement, the mixtures were made for all the different kinds of mixture at 180 °C. The higher melting temperature corresponds to the PHA at 175 °C and the lowest temperature of degradation at the SA at 180 °C. The speed of the mixture was determined according to previous work in our laboratory [
15]. First, the primary binder and the PEG were introduced in the mixer and then the SA and the powder. The mixture is homogenized when the mixing torque is stable.
The rheological tests were performed on a capillary rheometer Rosand RH2000 provided by Malvern Instruments (Malvern, UK). The tests are realized at 180 °C, the temperature selected for injection, in order to characterize the viscoelastic properties of the binders. The feedstocks were characterized at shear rate corresponding to the usual injection shear rate, between 2000 s−1 and 50,000 s−1 in a range of shear rates matching those encountered in injection process. The injection was realized on an injection machine (Arburg, Loßburg, Germany) at 180 °C. Cylinders of 100 mm of length and 10 mm of diameter were injected.
The choice of the PEG was made due to his possibility to be removed by water. To study this method of debinding, a cylinder of the different mixtures were injected. Different methods of water debinding were tested. First the samples are put on a bath of heat water [
16,
17]. Two different temperatures were tested, 50 °C and 60 °C, and the test were made during 24 h and 48 h because these conditions correspond to removal of the entire PEG from the feedstock made with the PP. Another test was made using a magnetic stirrer in the water bath [
16,
18]. To compare the result with the previous test, the same temperature and time were studied. Finally, an ultrasonic bath was used [
18]. The ultrasonic bath use a 35 KHz and 50 W ultrasonic waves. Images of the samples before and after debinding were also made to characterize the possible defaults which can appear during the different process. To determine the weight loss of PEG the samples are weighed before and after each test. This is an approximation but only the PEG is logically soluble in water so the results are close to reality. To confirm the results, some TGA on the samples have to be made.
According to the work of Federzoni
et al. [
14] a preliminary test was made at 70 °C, 300 bar during 4 h. However, a previous test on the samples injected with the feedstock with PP shows only 40% of weight loss of PEG. Another test at 150 °C and 400 bars during 4 h was made and the results were better. These conditions were choosen because, according to the data from the National Institute of Standards and Technology [
19], the density and the viscosity of the supercritical CO
2 are similar at 70 °C, 300 bar (257.34 kg/m
3, 26.004 µPa·s) and 150 °C, 400 bar (259.50 kg/m
3, 29.180 µPa·s). So the coefficient of diffusivity and solubility of the PEG in the CO
2 does not change because of the properties of the CO
2 but due to the increase of the temperature and the pressure that enhance these coefficients. The supercritical debinding was realized in a SFE2.2 supercritical fluid reactor provided by Separex (Champigneulles, France).
Acknowledgments
The authors wish to thank the FUI ProPIM project for the financial support.
Author Contributions
Alexandre Royer designed and performed the experiments. Alexandre Royer analyzed the data and wrote the paper. Thierry Barrière and Jean-Claude Gelin supervised the study and corrected the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PP | polypropylene |
| PEG | polyethylene glycol |
| PHA | polyhydroxyalkanoates |
| PHBV | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| SA | stearic acid |
| TGA | thermogravimetric analysis |
| MIM | metal injection moulding |
References
- German, R.M. Powder Injection Moulding—Design and Applications; Innovative Material Solution, Inc.: State College, PA, USA, 2003. [Google Scholar]
- Enneti, R.K.; Onbattuvelli, V.P.; Atre, S.V. Powder binder formulation and compound manufacture in metal injection molding (MIM). In Handbook of Metal Injection Molding; Heaney, D.F., Ed.; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Cambridge, UK, 2012; pp. 64–92. [Google Scholar]
- Quinard, C.; Song, J.; Barriere, T.; Gelin, J.C. Elaboration of PIM feedstocks with 316L fine stainless steel powders for the processing of micro-components. Powder Technol. 2011, 208, 383–389. [Google Scholar] [CrossRef]
- Tam, K.C.; Yap, S.P.; Foong, M.L.; Loh, N.H. Metal injection molding: Effects of the vinyl acetate content on binder behavior. J. Mater. Process. Technol. 1997, 67, 120–125. [Google Scholar] [CrossRef]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Sheth, M.; Kumar, R.A.; Davé, V.; Gross, R.A.; McCarthy, S.P. Biodegradable polymer blends of poly(lactic acid) and poly(ethylene glycol). J. Appl. Polym. Sci. 1997, 66, 1495–1505. [Google Scholar] [CrossRef]
- Castillo, R.V.; Müller, A.J. Crystallization and morphology of biodegradable or biostable single and double crystalline block copolymers. Prog. Polym. Sci. 2009, 34, 516–560. [Google Scholar] [CrossRef]
- Thavanayagam, G.; Pickering, K.L.; Swan, J.E.; Cao, P. Analysis of rheological behaviour of titanium feedstocks formulated with a water-soluble binder system for powder injection moulding. Powder Technol. 2015, 269, 227–232. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J. Biodegradable Polymers and Polymer Blends. In Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 109–128. [Google Scholar]
- Quinard, C.; Barriere, T.; Gelin, J.C. Development and property identification of 316L stainless steel feedstock for PIM and µPIM. Powder Technol. 2009, 190, 123–128. [Google Scholar] [CrossRef]
- Özgün, Ö.; Gülsoy, H.Ö.; Yılmaz, R.; Fındık, F. Microstructural and mechanical characterization of injection molded 718 superalloy powders. J. Alloy. Compd. 2013, 576, 140–153. [Google Scholar] [CrossRef]
- Chartier, T.; Ferrato, M.; Baumard, J.-F. Influence of the debinding method on the mechanical properties of plastic formed ceramics. J. Eur. Ceram. Soc. 1995, 15, 899–903. [Google Scholar] [CrossRef]
- Shimizu, T. Solvent debinding of injection moulded parts with supercritical carbon dioxide. Met. Powder Rep. 1997, 52, 41. [Google Scholar]
- Federzoni, L.; Revirand, P.; Lumia, D.; Rossignol, G. Evaluation of the supercritical CO2 as method of debinding for PIM parts. In Proceedings of the Internationnal Congress & Exibition, Euro PM, Copenhagen, Denmark, 12–14 October 2009.
- Djoudi, H.; Gelin, J.-C.; Barrière, T. Experiments and FE simulation for twin screw mixing of nanocomposite of polypropylene/multi-walled carbon nanotubes. Compos. Sci. Technol. 2015, 107, 169–176. [Google Scholar] [CrossRef]
- Chen, G.; Cao, P.; Wen, G.; Edmonds, N. Debinding behaviour of a water soluble PEG/PMMA binder for Ti metal injection moulding. Mater. Chem. Phys. 2013, 139, 557–565. [Google Scholar] [CrossRef]
- Hidalgo, J.; Jiménez-Morales, A.; Barriere, T.; Gelin, J.C.; Torralba, J.M. Water soluble Invar 36 feedstock development for μPIM. J. Mater. Process. Technol. 2014, 214, 436–444. [Google Scholar] [CrossRef]
- Shbeh, M.M.; Goodall, R. Design of water debinding and dissolution stages of metal injection moulded porous Ti foam production. Mater. Des. 2015, 87, 295–302. [Google Scholar] [CrossRef]
- US Department of Commerce. National Institute of Standards and Technology. Available online: http://www.nist.gov/ (accessed on 25 March 2016).
- Hidalgo, J.; Abajo, C.; Jiménez-Morales, A.; Torralba, J.M. Effect of a binder system on the low-pressure powder injection moulding of water-soluble zircon feedstocks. J. Eur. Ceram. Soc. 2013, 33, 3185–3194. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).