Decrease in Hydrogen Embrittlement Susceptibility of 10B21 Screws by Bake Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Twist-Off Strength and Hydrogen Embrittlement Testing
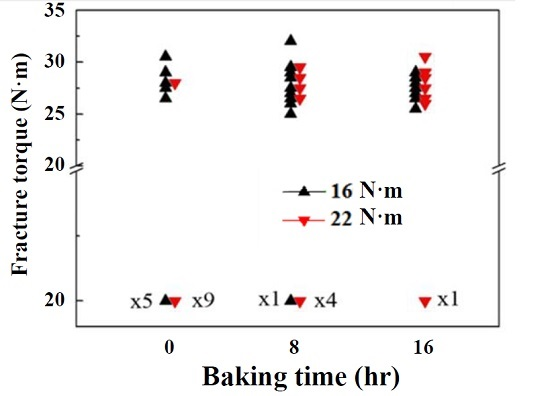
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 10B21 | low-carbon boron steel |
| HE | hydrogen embrittlement |
| SIMS | secondary ion mass spectroscopy |
| OM | optical microscope |
| HR-SEM | high-resolution scanning electron microscopy |
References
- Woodtli, J.; Kieselbach, R. Damage due to hydrogen embrittlement and stress cracking. Eng. Fail. Anal. 2000, 7, 427–450. [Google Scholar] [CrossRef]
- Rebak, R.B.; Muchjin, L.; Szklarska-Smialowska, Z. Hydrogen diffusion and accumulation in automotive fasteners. Corrosion 1997, 33, 481–488. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Revie, R.W. Corrosion and Corrosion Control, 3rd ed.; Wiley: New York, NY, USA, 1985; p. 274. [Google Scholar]
- Knott, J.K. Some new aspects of hydrogen-assisted crack growth in steels. J. Chin. Corros. Eng. 1995, 9, 215–224. [Google Scholar]
- Curry, D.A.; Knott, J.F. Effect of microstructure on cleavage fracture toughness of quenched and tempered steels. Met. Sci. 1979, 13, 341–345. [Google Scholar] [CrossRef]
- Yu, X.; Wen, S. Hydrogen-induced delayed failure of TWIP steel with vanadium addition. Shanghai Met. 2014, 36, 18–22. [Google Scholar]
- Bhadeshia, H.K.D.H. Prevention of hydrogen embrittlement in steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Kuruvilla, A.K.; Stoloff, N.S. Hydrogen embrittlement of Ni3Al+B. Scr. Metall. 1985, 19, 83–87. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y.; Matsuoka, S. Hydrogen embrittlement mechanism in fatigure of austenitic stainless steels. Metall. Mater. Trans. A 2008, 39, 1327–1339. [Google Scholar] [CrossRef]
- Hwang, B.; Suh, D.W.; Kim, S.J. Austenitizing temperature and hardenability of low-carbon boron steels. Scr. Mater. 2011, 64, 1118–1120. [Google Scholar] [CrossRef]
- Wittmaack, K. Background formation in SIMS analysis of hydrogen, carbon, nitrogen and oxygen in silicon. Nucl. Instrum. Methods 1983, 218, 327–332. [Google Scholar] [CrossRef]
- Morito, S.; Saito, H.; Owawa, T.; Furuhara, T.; Maki, T. Effect of austenite grain size on the morphology and crystallography of lath martensite in low carbon steels. ISIJ Int. 2005, 45, 91–94. [Google Scholar] [CrossRef]
- Fukuda, Y.; Oh-ishi, K.; Horita, Z.; Langdon, T.G. Processing of a low-carbon steel by equal-channel angular pressing. Acta Mater. 2002, 50, 1359–1368. [Google Scholar] [CrossRef]
- Hardie, D.; Charles, E.A.; Lopez, A.H. Hydrogen embrittlement of high strength pipeline steels. Corros. Sci. 2006, 48, 4378–4385. [Google Scholar] [CrossRef]
- Eastman, J.; Heubaum, F.; Matsumoto, T.; Birnbaum, H.K. The effect of hydrogen on the solid solution strengthening and softening of nickel. Acta Metall. 1982, 30, 1579–1986. [Google Scholar] [CrossRef]
- Hillier, E.M.K.; Robinson, M.J. Hydrogen embrittlement of high strength steel electroplated with zinc–cobalt alloys. Corros. Sci. 2004, 46, 715–727. [Google Scholar] [CrossRef] [Green Version]
- McMahon, C.J., Jr. Hydrogen-induced intergranular fracture of steels. Eng. Fract. Mech. 2001, 68, 773–788. [Google Scholar] [CrossRef]
- Wittmaack, K. Pre-equilibrium variation of the secondary ion yield. Int. J. Mass Spectrom. Ion Phys. 1975, 17, 39–50. [Google Scholar] [CrossRef]







| Fe | C | Mn | P | S | Si | Al | B |
|---|---|---|---|---|---|---|---|
| Bal. | 0.18 | 0.78 | 0.015 | 0.005 | 0.06 | 0.044 | 0.002 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-J.; Hung, F.-Y.; Lui, T.-S.; Tseng, C.-H. Decrease in Hydrogen Embrittlement Susceptibility of 10B21 Screws by Bake Aging. Metals 2016, 6, 211. https://doi.org/10.3390/met6090211
Chen K-J, Hung F-Y, Lui T-S, Tseng C-H. Decrease in Hydrogen Embrittlement Susceptibility of 10B21 Screws by Bake Aging. Metals. 2016; 6(9):211. https://doi.org/10.3390/met6090211
Chicago/Turabian StyleChen, Kuan-Jen, Fei-Yi Hung, Truan-Sheng Lui, and Chien-Hao Tseng. 2016. "Decrease in Hydrogen Embrittlement Susceptibility of 10B21 Screws by Bake Aging" Metals 6, no. 9: 211. https://doi.org/10.3390/met6090211