Influence of Intermetallic Particles on the Corrosion Properties of Extruded ZK60 Mg Alloy Containing Cu
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
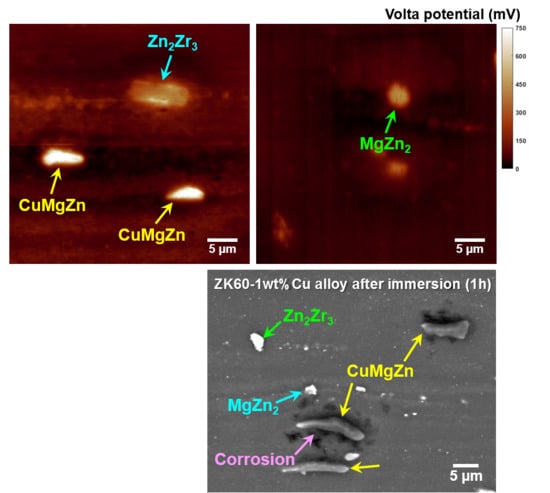
3.1. Microstructure
3.2. Corrosion Properties
3.3. Influence of Intermetallic Particles
3.4. Corrosion-Controlling Factor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bettles, C.J.; Gibson, M.A. Current wrought magnesium alloys: Strength and weakness. JOM 2005, 57, 46–49. [Google Scholar] [CrossRef]
- Kim, N.J. Magnesium sheet alloys: Viable alternatives to steels? Mater. Sci. Technol. 2014, 30, 1925–1928. [Google Scholar] [CrossRef]
- Davies, C.; Barnett, M. Expanding the extrusion limits of wrought magnesium alloys. JOM 2004, 56, 22–24. [Google Scholar] [CrossRef]
- Peng, Z.; Sheppard, T. Study of surface cracking during extrusion of aluminium alloy AA 2014. Mater. Sci. Technol. 2004, 20, 1179–1191. [Google Scholar] [CrossRef]
- Polmear, I.J. Light Alloys, 3rd ed.; Arnold: London, UK, 1995; pp. 219–221. ISBN 0-340-63207-0. [Google Scholar]
- Park, S.H.; Yu, H.; Kim, H.S.; Bae, J.H.; Yim, C.D.; You, B.S. Effect of Cu addition on the microstructure and mechanical properties of an as-extruded ZK60 alloy. Korean J. Met. Mater. 2013, 51, 169–179. [Google Scholar] [CrossRef]
- Buha, J. Mechanical properties of naturally aged Mg–Zn–Cu–Mn alloy. Mater. Sci. Eng. A 2008, 489, 127–137. [Google Scholar] [CrossRef]
- Zhu, H.M.; Sha, G.; Liu, J.W.; Wu, C.L.; Luo, C.P.; Liu, Z.W.; Zheng, R.K.; Ringer, S.P. Microstructure and mechanical properties of Mg–6Zn–xCu–0.6Zr (wt %) alloys. J. Alloy. Compd. 2011, 509, 3526–3531. [Google Scholar] [CrossRef]
- Yu, H.; Yu, H.S.; Kim, Y.M.; You, B.S.; Min, G.H. Hot deformation behavior and processing maps of Mg–Zn–Cu–Zr magnesium alloy. Trans. Nonferr. Met. Soc. 2013, 23, 756–764. [Google Scholar] [CrossRef]
- Zhu, H.M.; Luo, C.P.; Liu, J.W.; Jiao, D.L. Effects of Cu addition on microstructure and mechanical properties of as-cast magnesium alloy ZK60. Trans. Nonferr. Met. Soc. 2014, 24, 605–610. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Nelson, C.E.; Peloubet, J.A. Corrosion studies of magnesium and its alloys. Trans. AIME 1942, 147, 273–299. [Google Scholar]
- Hillis, J.E.; Reichek, K.N. High. Purity Magnesium AM60 Alloy.: The Critical Contaminant Limits and the Salt Water Corrosion Performance; SAE Technical Paper; Society of Automotive Engineers: Detroit, MI, USA, 1986; p. 860288. [Google Scholar]
- Song, G.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Budruk Abhijeet, S.; Balasubramaniam, R.; Gupta, M. Corrosion behavior of Mg–Cu and Mg–Mo composites in 3.5% NaCl. Corros. Sci. 2008, 50, 2423–2428. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, B.; Baek, S.-M.; Sohn, S.-D.; Shin, H.-J.; Jeong, H.Y.; Yim, C.D.; You, B.S.; Ha, H.-Y.; Park, S.S. Influence of alloyed Al on the microstructure and corrosion properties of extruded Mg–8Sn–1Zn alloys. Corros. Sci. 2015, 95, 133–142. [Google Scholar] [CrossRef]
- Baek, S.-M.; Kim, H.J.; Jeong, H.Y.; Sohn, S.-D.; Shin, H.-J.; Choi, K.-J.; Lee, K.-S.; Lee, J.G.; Yim, C.D.; You, B.S.; et al. Effect of alloyed Ca on the microstructure and corrosion properties of extruded AZ61 Mg alloy. Corros. Sci. 2016, 112, 44–53. [Google Scholar] [CrossRef]
- Galiyev, A.; Kaibyshev, R.; Gottstein, G. Correlation of plastic deformation and dynamic recrystallization in magnesium alloy ZK60. Acta Mater. 2001, 49, 1199–1207. [Google Scholar] [CrossRef]
- Ma, C.J.; Liu, M.; Wu, G.H.; Ding, W.J.; Zhu, Y.P. Microstructure and mechanical properties of extruded ZK60 magnesium alloy containing rare earth. Mater. Sci. Technol. 2004, 20, 1661–1665. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Rong, J.; Yu, Z.-Y.; Zha, M.; Wang, C.; Yang, Z.-Z.; Bu, R.-Y.; Jiang, Q.-C. Tensile properties, texture evolution and deformation anisotropy of as-extruded Mg–6Zn–1Zr magnesium alloy at room and elevated temperatures. Mater. Sci. Eng. A 2017, 697, 149–157. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Jiang, L. Evolution of precipitates in ZK60 magnesium alloy during high strain rate deformation. J. Alloy. Compd. 2017, 705, 566–571. [Google Scholar] [CrossRef]
- De Wit, J.H.W. Local potential measurements with the SKPFM on aluminium alloys. Electrochim. Acta 2004, 49, 2841–2850. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Susceptibility of rare-earth-magnesium alloys to micro-galvanic corrosion. Corros. Sci. 2010, 52, 3896–3906. [Google Scholar] [CrossRef]
- Hurley, M.F.; Efaw, C.M.; Davis, P.H.; Croteau, J.R.; Graugnard, E.; Birbilis, N. Volta potentials measured by scanning Kelvin probe force microscopy as relevant to corrosion of magnesium alloys. Corrosion 2015, 71, 160–170. [Google Scholar] [CrossRef]
- Esmaily, M.; Blücher, D.B.; Svensson, J.E.; Halvarsson, M.; Johansson, L.G. New insights into the corrosion of magnesium alloys—The role of aluminum. Scr. Mater. 2016, 115, 91–95. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kim, H.J.; Baek, S.-M.; Kim, B.; Sohn, S.-D.; Shin, H.-J.; Jeong, H.Y.; Park, S.H.; Yim, C.D.; You, B.S.; et al. Improved corrosion resistance of extruded Mg–8Sn–1Zn–1Al alloy by microalloying with Mn. Scr. Mater. 2015, 109, 38–43. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg–5 wt % Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar] [CrossRef]
- Birbilis, N.; Williams, G.; Gusieva, K.; Samaniego, A.; Gibson, M.A.; McMurray, H.N. Poisoning the corrosion of magnesium. Electrochem. Commun. 2013, 34, 295–298. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Yang, J.; Yim, C.D.; You, B.S. Limitations in the use of the potentiodynamic polarisation curves to investigate the effect of Zn on the corrosion behaviour of as-extruded Mg–Zn binary alloy. Corros. Sci. 2013, 75, 426–433. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Kim, S.G.; Kim, B.; Park, S.S.; Yim, C.D.; You, B.S. Influences of metallurgical factors on the corrosion behaviour of extruded binary Mg–Sn alloys. Corros. Sci. 2014, 82, 369–379. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Yim, C.D.; Yang, J.; You, B.S. Role of hydrogen evolution rate in determining the corrosion rate of extruded Mg–5Sn–(1–4 wt %)Zn alloys. Corros. Sci. 2014, 89, 275–285. [Google Scholar] [CrossRef]
- Baek, S.-M.; Kang, J.S.; Shin, H.-J.; Yim, C.D.; You, B.S.; Ha, H.-Y.; Park, S.S. Role of alloyed Y in improving the corrosion resistance of extruded Mg–Al–Ca-based alloy. Corros. Sci. 2017, 118, 227–232. [Google Scholar] [CrossRef]
- Cao, F.; Song, G.-L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef]





| Alloy | Zn | Zr | Cu | Fe | Si | Mg |
|---|---|---|---|---|---|---|
| ZK60 | 5.50 | 0.58 | <0.001 | 0.0026 | 0.0019 | bal. |
| ZKC601 | 5.52 | 0.57 | 0.88 | 0.0029 | 0.0018 | bal. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.-M.; Kim, B.; Park, S.S. Influence of Intermetallic Particles on the Corrosion Properties of Extruded ZK60 Mg Alloy Containing Cu. Metals 2018, 8, 323. https://doi.org/10.3390/met8050323
Baek S-M, Kim B, Park SS. Influence of Intermetallic Particles on the Corrosion Properties of Extruded ZK60 Mg Alloy Containing Cu. Metals. 2018; 8(5):323. https://doi.org/10.3390/met8050323
Chicago/Turabian StyleBaek, Soo-Min, Beomcheol Kim, and Sung Soo Park. 2018. "Influence of Intermetallic Particles on the Corrosion Properties of Extruded ZK60 Mg Alloy Containing Cu" Metals 8, no. 5: 323. https://doi.org/10.3390/met8050323