Green Activated Magnetic Graphitic Carbon Oxide and Its Application for Hazardous Water Pollutants Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphitic Carbon Preparation
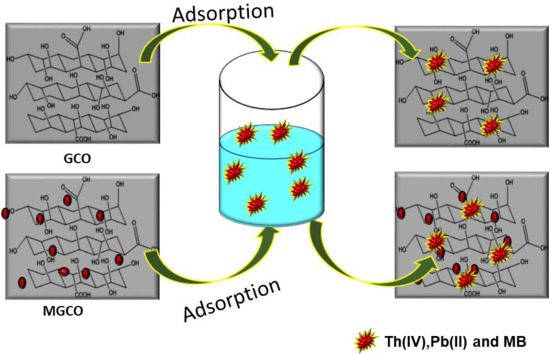
2.2. Graphitic Carbon Oxide (GCO) Preparation
2.3. Magnetic Graphitic Carbon Oxide (MGCO) Preparation
2.4. The Adsorptive Procedure of GCO and MGCO for Removal of Pollutants
3. Results and Discussion
3.1. Optimization of the Ozonation Process with Solution pH
3.2. Experimental Parameters for Adsorptive Removal of Pollutants by MGCO and GCO
3.3. Adsorption Isotherms
3.4. Reusability of MGCO
3.5. Characterization of Texture and Surface of Adsorbents and Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Novotny, V. Water Quality: Prevention, Identification and Management of Diffuse Pollution; Van Nostrand-Reinhold Publishers: New York, NY, USA, 1994. [Google Scholar]
- Lingamdinne, L.P.; Singh, J.; Choi, J.S.; Chang, Y.Y.; Yang, J.K.; Rama, K.R.; Koduru, J.R. Multivariate modeling via artificial neural network applied to enhance methylene blue sorption using graphene-like carbon material prepared from edible sugar. J. Mol. Liq. 2018, 265, 416–427. [Google Scholar] [CrossRef]
- Herrmann, S.; De Matteis, L.; de la Fuente, J.M.; Mitchell, S.G.; Streb, C. Removal of multiple contaminants from water by polyoxometalate supported ionic liquid phases (POM-SILPs). Angew. Chem. Int. Ed. 2017, 56, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Metaxas, M.; Kasselouri-Rigopoulou, V.; Galiatsatou, P.; Konstantopoulou, C.; Oikonomou, D. Thorium removal by different adsorbents. J. Hazard. Mater. 2003, 97, 71–82. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Chang, Y.Y.; Yang, J.K.; Singh, J.; Choi, E.A.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 2017, 307, 74–84. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Jyothi, R.K.; Chang, Y.Y.; Yang, J.K. Factors affect on bioremediation of Co(II) and Pb(II) onto Lonicera japonica flowers powder. Desalin. Water Treat. 2016, 57, 13066–13080. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Ground Water and Drinking Water, Table of Regulated Drinking Water Contaminants. Available online: https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-watercontaminants#Inorganic2016 (accessed on 1 May 2017).
- Konicki, W.; Sibera, D.; Mijowska, E.; Lendzion-Bielun, Z.; Narkiewicz, U. Equilibrium and kinetic studies on acid dye acid red 88 adsorption by magnetic ZnFe2O4 spinel ferrite nanoparticles. J. Colloid Interface Sci. 2013, 398, 152–160. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Awual, M.R. Assessing of lead(II) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem. Eng. J. 2016, 289, 65–73. [Google Scholar] [CrossRef]
- Hummers, S.W.; Offeman, R.E., Jr. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lakshmi, P.L.; Janardhan, R.K. Magnetic graphene oxide composites are the solutions for sustainable remediation of ecosystems. Environ. Anal. Ecol. Stud. 2018, 2, 528. [Google Scholar]
- Mishra, A.K.; Ramaprabhu, S. Functionalized graphene sheets for arsenic removal and desalination of sea water. Desalination 2011, 282, 39–45. [Google Scholar] [CrossRef]
- Zabihi, M.; Ahmadpour, A.; Asl, A.H. Removal of mercury from water by carbonaceous sorbents derived from walnut shell. J. Hazard. Mater. 2009, 167, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Choi, Y.L.; Kim, I.S.; Chang, Y.Y.; Koduru, J.R.; Yang, J.K. Porous graphene oxide based inverse spinel nickel ferrite nanocomposites for the enhanced adsorption removal of arsenic. RSC Adv. 2016, 6, 73776–73789. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Roh, H.; Choi, Y.L.; Chang, Y.Y.; Yang, J.K. Adsorption removal of Co(II) from waste-water using graphene oxide. Hydrometallurgy 2016, 165, 90–96. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Zhai, Y.; Wang, E. Nafion–graphene nanocomposite film as enhanced sensing platform for ultrasensitive determination of cadmium. Electrochem. Commun. 2009, 11, 1085–1088. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye adsorption on layered graphite oxide. J. Chem. Eng. Data 2010, 56, 138–141. [Google Scholar] [CrossRef]
- Wang, C.; Feng, C.; Gao, Y.; Ma, X.; Wu, Q.; Wang, Z. Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem. Eng. J. 2011, 173, 92–97. [Google Scholar] [CrossRef]
- Choi, J.S.; Koduru, J.R.; Lingamdinne, L.P.; Yang, J.K.; Chang, Y.Y. Effective adsorptive removal of 2,4,6-trinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine by pseudographitic carbon: Kinetics, equilibrium and thermodynamics. Environ. Chem. 2018, 15, 100–112. [Google Scholar] [CrossRef]
- Tao, H.; Moser, J.; Alzina, F.; Wang, Q.; Sotomayor-Torres, C.M. The morphology of graphene sheets treated in an ozone generator. J. Phys. Chem. 2011, 115, 18257–18260. [Google Scholar] [CrossRef]
- Huh, S.; Park, J.; Kim, Y.S.; Kim, K.S.; Hong, B.H.; Nam, J.M. UV/ozone-oxidized large-scale graphene platform with large chemical enhancement in surface-enhanced Raman scattering. ACS Nano 2011, 5, 9799. [Google Scholar] [CrossRef]
- Larciprete, R.; Lacovig, P.; Gardonio, S.; Baraldi, A.; Lizzit, S. Atomic oxygen on graphite: chemical characterization and thermal reduction. J. Phys. Chem. C 2012, 116, 9900–9908. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Cheng, Y.C.; Faccio, R.; Schwingenschlogl, U. Oxidation of monovacancies in graphene by oxygen molecules. J. Mater. Chem. 2011, 21, 18284. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Kaloni, T.P.; Zhu, Z.Y.; Schwingenschlogl, U. Oxidation of graphene in ozone under ultraviolet light. Appl. Phys. Lett. 2012, 101, 073110. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.L.; Choi, J.S.; Lingamdinne, L.P.; Chang, Y.Y.; Koduru, J.R.; Ha, J.H.; Yang, J.K. Removal of U(VI) by sugar-based magnetic pseudo–graphene oxide and its application to authentic groundwater using electromagnetic system. Environ. Sci. Pollut. Res. 2019, 26, 22323–22337. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological aspects of ozone applications in food: A review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Wilhelm, A.E.; Goulart, I.B.; Tonon, R.V.; Freitas-Silva, O.; Germani, R.; Chávez, D.W.H. Effect of water temperature and pH on the concentration and time of ozone saturation. Braz. J. Food Technol. 2018, 21, e2017156. [Google Scholar] [CrossRef]
- Jung, Y.; Hong, E.; Kwon, M.; Kang, J.W. A kinetic study of ozone decay and bromine formation in saltwater ozonation: Effect of O3 dose, salinity, pH and temperature. Chem. Eng. J. 2017, 312, 30–38. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Zhang, P.; Ning, J.; Li, J.; Su, Z.; Wei, G. A facile fabrication of large-scale reduced graphene oxide–silver nanoparticle hybrid film as a highly active surface-enhanced Raman scattering substrate. J. Mater. Chem. C 2015, 3, 4126–4133. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, X.Y.; Ma, Y.F.; Huang, Y.; Wang, Y.S.; Chen, Y.S. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 2710. [Google Scholar] [CrossRef]
- Xuan, S.H.; Jiang, W.Q.; Gong, X.L.; Hu, Y.; Chen, Z.Y. Magnetically separable Fe3O4/TiO2 hollow spheres: Fabrication and photocatalytic activity. J. Phys. Chem. C 2009, 113, 553. [Google Scholar] [CrossRef]










| Name of Adsorbent | Pollutant | qe, Th, mg·g−1 | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|---|
| qe, Cal., mg·g−1 | K1 | R2 | qe, Cal., mg·g−1 | K2 | R2 | |||
| GCO | Th(IV) | 19.05 | 23.47 | 0.32 | 0.818 | 18.38 | 0.031 | 0.999 |
| Pb(II) | 18.95 | 11.035 | 0.034 | 0.925 | 19.01 | 0.005 | 0.998 | |
| MB | 38.85 | 49.97 | 0.15 | 0.910 | 37.34 | 0.055 | 0.999 | |
| MGCO | Pb(II) | 23.05 | 13.00 | 0.037 | 0.912 | 23.31 | 0.004 | 0.999 |
| MB | 32.56 | 25.62 | 0.149 | 0.845 | 33.34 | 0.047 | 1 | |
| Name of Adsorbent | Pollutant | Langmuir | Freundlich | Temkin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax, mg·g−1 | KL, L mg−1 | R2 | χ2 | KF, mg·g−1 (L·mg−1)1/n | n | R2 | χ2 | B, g·L−1 | KT, L·mg−1 | R2 | χ2 | ||
| GCO | Th(IV) | 52.63 | 0.73 | 0.991 | 25.69 | 20.47 | 2.20 | 0.924 | 45.69 | 40.49 | 15.59 | 0.966 | 32.65 |
| Pb(II) | 47.39 | 0.86 | 0.993 | 19.65 | 16.95 | 2.23 | 0.954 | 52.65 | 20.11 | 11.63 | 0.952 | 42.12 | |
| MB | 111.12 | 1.10 | 0.997 | 22.36 | 52.48 | 2.67 | 0.916 | 39.65 | 20.60 | 120.60 | 0.914 | 40.25 | |
| MGCO | Th(IV) | 76.02 | 0.89 | 0.991 | 18.56 | 30.88 | 1.67 | 0.861 | 38.69 | 22.15 | 14.30 | 0.925 | 35.64 |
| Pb(II) | 71.94 | 0.92 | 0.993 | 28.96 | 27.45 | 1.73 | 0.830 | 42.58 | 32.31 | 12.63 | 0.912 | 40.25 | |
| MB | 76.92 | 0.30 | 0.991 | 22.36 | 47.02 | 4.03 | 0.890 | 36.65 | 32.37 | 65.80 | 0.882 | 38.79 | |
| Parameters | GC | GCO | MGCO |
|---|---|---|---|
| BET Surface Area (m2·g−1) | 674.593 | 655.894 | 428.382 |
| Langmuir Surface Area (m2·g−1) | 746.798 | 728.914 | 506.278 |
| Pore volume (cm3·g−1) | 0.278 | 0.259 | 0.146 |
| Average pore diameter (nm) | 1.647 | 2.181 | 5.223 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lingamdinne, L.P.; Choi, J.-S.; Choi, Y.-L.; Yang, J.-K.; Koduru, J.R.; Chang, Y.-Y. Green Activated Magnetic Graphitic Carbon Oxide and Its Application for Hazardous Water Pollutants Removal. Metals 2019, 9, 935. https://doi.org/10.3390/met9090935
Lingamdinne LP, Choi J-S, Choi Y-L, Yang J-K, Koduru JR, Chang Y-Y. Green Activated Magnetic Graphitic Carbon Oxide and Its Application for Hazardous Water Pollutants Removal. Metals. 2019; 9(9):935. https://doi.org/10.3390/met9090935
Chicago/Turabian StyleLingamdinne, Lakshmi Prasanna, Jong-Soo Choi, Yu-Lim Choi, Jae-Kyu Yang, Janardhan Reddy Koduru, and Yoon-Young Chang. 2019. "Green Activated Magnetic Graphitic Carbon Oxide and Its Application for Hazardous Water Pollutants Removal" Metals 9, no. 9: 935. https://doi.org/10.3390/met9090935