High Performance Asymmetric Supercapacitor Based on Hierarchical Carbon Cloth In Situ Deposited with h-WO3 Nanobelts as Negative Electrode and Carbon Nanotubes as Positive Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of WO3/CC
2.2. Preparation of CNT/CC
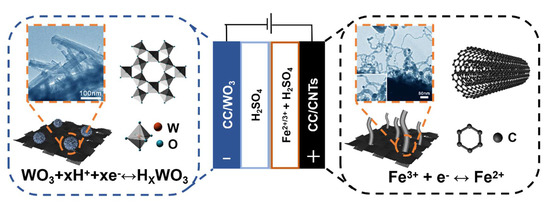
2.3. Assembly of the Asymmetric Supercapacitor
2.4. Characterizations
3. Results and Discussions
3.1. The Structure and Electrochemical Behavior of the WO3/CC Electrode
3.2. The Structure and Electrochemical Performance of CNT/CC
3.3. The Electrochemical Performance of the Assembled Asymmetric Supercapacitor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Sung, J.; Shin, C. Recent studies on supercapacitors with next-generation structures. Micromachines 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Saeed, G.; Alam, A.; Bandyopadhyay, P.; Lim, S.; Kim, N.H.; Lee, J.H. Development of hierarchically structured nanosheet arrays of CuMnO2-MnxOy@graphene foam as a nanohybrid electrode material for high-performance asymmetric supercapacitor. J. Alloys Compd. 2021, 858, 158343. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Y.; Peng, J.; Yang, J.; Wu, C.; Chang, B.; Guo, X.; Chen, G.; Luo, Z.; Wang, X. Nickel sulfide/activated carbon nanotubes nanocomposites as advanced electrode of high-performance aqueous asymmetric supercapacitors. J. Alloys Compd. 2021, 885, 160979. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, F.; Zhang, Z.; Zhuang, Q.; Yu, H.; Huang, Y.; Liu, Q.; Fu, M. Synthesis of the cathode and anode materials from discarded surgical masks for high-performance asymmetric supercapacitors. J Colloid Interface Sci. 2021, 603, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Khan, M.I.; Cheng, F.; Lu, W. A review on selection criteria of aqueous electrolytes performance evaluation for advanced asymmetric supercapacitors. J. Energy Storage 2021, 40, 102729. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef]
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Sabahi Namini, A.; Le, Q.V.; Shokouhimehr, M.; Shahedi Asl, M. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2021, 857, 158281. [Google Scholar] [CrossRef]
- Amar, V.S.; Houck, J.D.; Maddipudi, B.; Penrod, T.A.; Shell, K.M.; Thakkar, A.; Shende, A.R.; Hernandez, S.; Kumar, S.; Gupta, R.B. Hydrothermal liquefaction (HTL) processing of unhydrolyzed solids (UHS) for hydrochar and its use for asymmetric supercapacitors with mixed (Mn,Ti)-Perovskite oxides. Renew. Energy 2021, 173, 329–341. [Google Scholar] [CrossRef]
- Xia, Z.; Mishukova, V.; Sollami Delekta, S.; Sun, J.; Sanchez, J.S.; Li, J.; Palermo, V. Selective deposition of metal oxide nanoflakes on graphene electrodes to obtain high-performance asymmetric micro-supercapacitors. Nanoscale 2021, 13, 3285–3294. [Google Scholar] [CrossRef]
- Chu, J.; Lu, D.; Wang, X.; Wang, X.; Xiong, S. WO3 nanoflower coated with graphene nanosheet: Synergetic energy storage composite electrode for supercapacitor application. J. Alloys Compd. 2017, 702, 568–572. [Google Scholar] [CrossRef]
- Jia, J.; Liu, X.; Mi, R.; Liu, N.; Xiong, Z.; Yuan, L.; Wang, C.; Sheng, G.; Cao, L.; Zhou, X.; et al. Self-assembled pancake-like hexagonal tungsten oxide with ordered mesopores for supercapacitors. J. Mater. Chem. A 2018, 6, 15330–15339. [Google Scholar] [CrossRef]
- Lokhande, V.; Lokhande, A.; Namkoong, G.; Kim, J.H.; Ji, T. Charge storage in WO3 polymorphs and their application as supercapacitor electrode material. Results Phys. 2019, 12, 2012–2020. [Google Scholar] [CrossRef]
- Shinde, P.A.; Seo, Y.; Ray, C.; Jun, S.C. Direct growth of WO3 nanostructures on multi-walled carbon nanotubes for high-performance flexible all-solid-state asymmetric supercapacitor. Electrochim. Acta 2019, 308, 231–242. [Google Scholar] [CrossRef]
- Kong, L.; Guo, X.; Xu, J.; Mo, Z.; Li, L. Morphology control of WO3 nanoplate film on W foil by oxalic acid for photocatalytic gaseous acetaldehyde degradation. J. Photochem. Photobiol. A Chem. 2020, 401, 112760. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, L.; Cai, Q.; Li, Q.; Gao, B.; Zhang, X.; Huo, K.; Chu, P.K. High-energy lithium-ion hybrid supercapacitors composed of hierarchical urchin-like WO3/C anodes and MOF-derived polyhedral hollow carbon cathodes. Nanoscale 2016, 8, 16761–16768. [Google Scholar] [CrossRef]
- Gu, Y.; Zheng, W.; Bu, Y. Facile preparation of nanoflower structured WO3 thin film on etched titanium substrate with high photoelectrochemical performance. J. Electroanal. Chem. 2019, 833, 54–62. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Reduced graphene oxide three-dimensionally wrapped WO3 hierarchical nanostructures as high-performance solar photocatalytic materials. Appl. Catal. A Gen. 2016, 522, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Shim, H.-W.; Lee, C.W.; Song, H.J.; Kim, J.-C.; Kim, D.-W. High-power and long-life supercapacitive performance of hierarchical, 3-D urchin-like W18O49 nanostructure electrodes. Nano Res. 2015, 9, 633–643. [Google Scholar] [CrossRef]
- Wang, Y.J.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Progress in modified carbon support materials for Pt and Pt-alloy cathode catalysts in polymer electrolyte membrane fuel cells. Prog. Mater. Sci. 2016, 82, 445–498. [Google Scholar] [CrossRef]
- Fang, B.; Kim, M.; Kim, J.H.; Yu, J.-S. Controllable synthesis of hierarchical nanostructured hollow core/mesopore shell carbon for electrochemical hydrogen storage. Langmuir 2008, 24, 12068–12072. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Fan, S.Q.; Kim, J.H.; Kim, M.S.; Kim, M.; Chaudhari, N.K.; Ko, J.; Yu, J.S. Incorporating hierarchical nanostructured carbon counter electrode into metal-free organic dye-sensitized solar cell. Langmuir 2010, 26, 11238–11243. [Google Scholar] [CrossRef] [PubMed]
- Gurten Inal, I.I.; Aktas, Z. Enhancing the performance of activated carbon based scalable supercapacitors by heat treatment. Appl. Surf. Sci. 2020, 514, 145895. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, P.; Feng, J.; Liu, Y.; Wang, L.; Liu, J.; Shi, X. Tailoring defective vanadium pentoxide/reduced graphene oxide electrodes for all-vanadium-oxide asymmetric supercapacitors. Chem. Eng. J. 2021, 429, 132274. [Google Scholar] [CrossRef]
- Han, X.; Huang, Z.-H.; Meng, F.; Jia, B.; Ma, T. Redox-etching induced porous carbon cloth with pseudocapacitive oxygenic groups for flexible symmetric supercapacitor. J. Energy Chem. 2022, 64, 136–143. [Google Scholar] [CrossRef]
- Li, G.R.; Xu, H.; Lu, X.F.; Feng, J.X.; Tong, Y.X.; Su, C.Y. Electrochemical synthesis of nanostructured materials for electrochemical energy conversion and storage. Nanoscale 2013, 5, 4056–4069. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, Y.; Liu, Y.; Sun, Z.; Cui, P.; Zhang, J.; Hua, X.; Su, Q.; Fu, J.; Xie, E. Energizing Fe2O3-based supercapacitors with tunable surface pseudocapacitance via physical spatial-confining strategy. Chem. Eng. J. 2021, 406, 126875. [Google Scholar] [CrossRef]
- Mo, Y.; Meng, W.; Xia, Y.; Du, X.; Lin, Z.; Li, W. Facile flame deposit of CNFs/Fe2O3 coating on 304 stainless steel mesh and their high capacitive performance. Electrochim. Acta 2020, 335, 135527. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Liu, P. Redox electroactive group-modified carbon cloth as flexible electrode for high performance solid-state supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124388. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Long, X.; Yang, M.; Song, X.; Xie, W.; Liu, D.; Fu, Y.; Li, J.; Li, Y.; et al. Cracked bark-inspired ternary metallic sulfide (NiCoMnS4) nanostructure on carbon cloth for high-performance aqueous asymmetric supercapacitors. Sci. China Mater. 2021, 64, 1632–1641. [Google Scholar] [CrossRef]
- Gupta, S.P.; More, M.A.; Late, D.J.; Walke, P.S. High-rate quasi-solid-state hybrid supercapacitor of hierarchical flowers of hydrated tungsten oxide nanosheets. Electrochim. Acta 2021, 366. [Google Scholar] [CrossRef]
- Sun, W.; Yeung, M.T.; Lech, A.T.; Lin, C.W.; Lee, C.; Li, T.; Duan, X.; Zhou, J.; Kaner, R.B. High surface area tunnels in hexagonal WO3. Nano Lett 2015, 15, 4834–4838. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.P.; Nishad, H.H.; Patil, V.B.; Chakane, S.D.; More, M.A.; Late, D.J.; Walke, P.S. Morphology and crystal structure dependent pseudocapacitor performance of hydrated WO3 nanostructures. Mater. Adv. 2020, 1, 2492–2500. [Google Scholar] [CrossRef]
- Mo, Y.; Zhou, H.; Zhang, B.; Du, X.; Lin, Z.; Li, W.; Liu, H.Y.; Mai, Y.-W. Facile flame catalytic growth of carbon nanomaterials on the surface of carbon nanotubes. Appl. Surf. Sci. 2019, 465, 23–30. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.Y.; Cai, G.; Mai, Y.W.; Baji, A. Use of facile mechanochemical method to functionalize carbon nanofibers with nanostructured polyaniline and their electrochemical capacitance. Nanoscale Res. Lett. 2012, 7, 111. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Luo, T.; Yang, C.; Yang, X.; Yang, S.; Cao, B. Laser assisted self-assembly synthesis of porous hollow MoO3-x-doped MoS2 nanospheres sandwiched by graphene for flexible high-areal supercapacitors. Electrochim. Acta 2020, 332, 135499. [Google Scholar] [CrossRef]
- Nie, G.; Zhao, X.; Jiang, J.; Luan, Y.; Shi, J.; Liu, J.; Kou, Z.; Wang, J.; Long, Y.-Z. Flexible supercapacitor of high areal performance with vanadium/cobalt oxides on carbon nanofibers as a binder-free membrane electrode. Chem. Eng. J. 2020, 402, 126294. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, M.; Liu, T.; Li, T.; Guo, D.; Liu, X.X. Cobalt-containing nanoporous nitrogen-doped carbon nanocuboids from zeolite imidazole frameworks for supercapacitors. Nanomaterials 2019, 9, 1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaie, K.; Scholz, F. Realizing alkaline all-pseudocapacitive supercapacitors based on highly stable nanospinel oxide coatings. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 576–582. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Nagaraju, G.; Yu, J.S. Conductive silver nanowires-fenced carbon cloth fibers-supported layered double hydroxide nanosheets as a flexible and binder-free electrode for high-performance asymmetric supercapacitors. Nano Energy 2017, 36, 58–67. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, H.; Zhu, C.; Lin, S.; Xu, Z.; Zhang, X. Free-standing MXene film modified by amorphous FeOOH quantum dots for high-performance asymmetric supercapacitor. Electrochim. Acta 2019, 308, 1–8. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Du, X. High Performance Asymmetric Supercapacitor Based on Hierarchical Carbon Cloth In Situ Deposited with h-WO3 Nanobelts as Negative Electrode and Carbon Nanotubes as Positive Electrode. Micromachines 2021, 12, 1195. https://doi.org/10.3390/mi12101195
Lin J, Du X. High Performance Asymmetric Supercapacitor Based on Hierarchical Carbon Cloth In Situ Deposited with h-WO3 Nanobelts as Negative Electrode and Carbon Nanotubes as Positive Electrode. Micromachines. 2021; 12(10):1195. https://doi.org/10.3390/mi12101195
Chicago/Turabian StyleLin, Jianhao, and Xusheng Du. 2021. "High Performance Asymmetric Supercapacitor Based on Hierarchical Carbon Cloth In Situ Deposited with h-WO3 Nanobelts as Negative Electrode and Carbon Nanotubes as Positive Electrode" Micromachines 12, no. 10: 1195. https://doi.org/10.3390/mi12101195