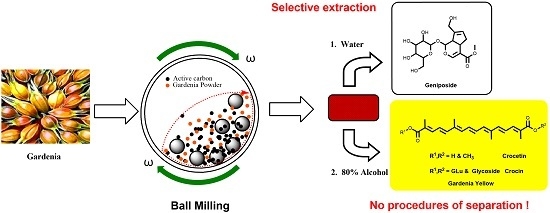
Selective Extraction of Gardenia Yellow and Geniposide from Gardenia jasminoides by Mechanochemistry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Ball-Milling Process
2.2. Optimization of Water Extraction Process
2.3. Optimization of Organic Solvent Extraction Process
2.4. Contrastive Analysis on Extract Methods of Gardenia Yellow
2.5. Possible Mechanism of Increasing Extraction Yield And Selective Gardenia Yellow Extraction
3. Materials and Methods
3.1. Preparation of Raw Materials
3.2. Chemicals and Reagents
3.3. Ball-Milling Process
3.4. Ultraviolet/Visible Spectrophotography Assay of Gardenia Yellow
| ABS | Absorbance | |
| S | Slope factor from the standard curve (S = 0.0894) | |
| I | Intercept factor from the standard curve (I = 0.0145) | |
| D | Dilution factor used for diluting the extracts to reach an absorbance in the range 0.4–0.7 | |
| Vextract | Amount (mL) of extract solution | |
| mgardenia | Amount (g) of pretreated gardenia fruits used for the extraction | |
| 106 | Factor to convert the results from μg/g to g/g | |
| GY% | Extraction yield of gardenia yellow from gardenia fruits (g/g) | |
| % MC | Moisture content of the pretreated gardenia fruits used for extraction (%, g/g) |
| ABS | Absorbance | |
| c | Concentration of gardenia yellow solution (g/mL) | |
| (440 nm ± 5 nm) | Color Value |
3.5. HPLC Assay of Geniposide
| PA | Peak Area of geniposide. |
| S | Slope from the standard curve (S = 0.0541) |
| I | Intercept from the standard curve (I = 0.225) |
| D | Dilution factor used for diluting the extracts to a reach peak area in the range 100–1000. |
| Vextract | Amount (mL) of extract solution. |
| mgardenia | Amount (g) of pretreated gardenia fruits used for the extraction. |
| 106 | Factor to convert the results from μg/g to g/g |
| G% | Extraction yield of geniposide from gardenia fruits (g/g) |
| % MC | Moisture content of the pretreated gardenia fruits used for extraction (%, g/g) |
3.6 Measurement of BET Surface Area and Observation of Microscopic Structure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GY% | Extraction rate of gardenia yellow |
| G% | Extraction rate of geniposide |
| MC | Moisture content |
| SEM | Scanning Electron microscope |
| SDS | Sodium dodecyl sulphate |
| CTAB | Hexadecyltrimethylammonium bromide |
| Tween 20 | Polyoxyethylene(20)Sorbitan Monolaurate |
References
- Xu, X. Traditional Chinese Medicine Decoction for Treating Angina. Chin. Patent CN 104367954 A, 25 February 2015. [Google Scholar]
- Yi, J. Traditional Chinese Medicine Wine for Treating Jaundice. Chin. Patent CN 104383144 A, 7 November 2015. [Google Scholar]
- Cai, M.-T.; Zuo, Y.-M.; Zhang, Z.-L.; Luo, G.-M. Chemical components of Gardeniae Fructus (IV). Chin. J. Exp. Trad. Med. Formulae 2014, 22, 88–91. [Google Scholar]
- Chen, H.; Xiao, Y.Q.; Li, L.; Zhang, C. Studies on chemical constituents in fruit of Gardenia jasminoides. China J. Chin. Materia Medica 2007, 32, 1041–1043. [Google Scholar]
- Coran, S.A.; Mulas, S.; Vasconi, A. Profiling of components and validated determination of iridoids in Gardenia Jasminoides Ellis fruit by a high-performance-thin-layer- chromatography/mass spectrometry approach. J. Chromatogr. A 2014, 1325, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kitamura, H.; Chino, M.; Takei, Y.; Hiruma, M.; Nomura, M. A 13-week oral dose subchronic toxicity study of gardenia yellow containing geniposide in rats. Food Chem. Toxicol. 2007, 45, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, J.; Li, X.; Du, Z. A new method research for determination of natural pigment crocin yellow in foods by solid-phase extraction ultrahigh pressure liquid chromatography. J. Chromatogr. A 2011, 1218, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, B.; Mansouri, M.T.; Ghorbanzadeh, B. Protective effects of crocin against streptozotocin-induced oxidative damage in rat striatum. Acta Med. Iran. 2014, 52, 101–105. [Google Scholar] [PubMed]
- Rezaee, R.; Mahmoudi, M.; Abnous, K.; Rabe, S.Z.T.; Tabasi, N.; Hashemzaei, M.; Karimi, G. Cytotoxic effects of crocin on MOLT-4 human leukemia cells. J. Complement. Integr. Med. 2013, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hassani, F.V.; Naseri, V.; Razavi, B.M.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF and VGF in rat hippocampus. DARU J. Pharm. Sci. 2014, 22. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Wang, L.Z.; Sun, L.R.; Dong, Q. Crocin attenuates cisplatin-induced liver injury in the mice. Hum. Exp. Toxicol. 2014, 33, 855–862. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, L.; Duan, L.; Cai, X.; Zhang, R. Nature Crocin Extraction Separation Method and Preparation for Crocin Blood Fat Reduction Drug. Chin. Patent CN 103665059 A, 26 March 2014. [Google Scholar]
- Zhu, X.; Mang, Y.; Shen, F.; Xie, J.; Su, W. Homogenate extraction of gardenia yellow pigment from Gardenia Jasminoides Ellis fruit using response surface methodology. J. Food Sci. Technol. 2014, 51, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, S.; Chen, F. Ultrasonic-assisted extraction of gardenia yellow from fructus Gardeniae. Adv. Mater. Res. 2012, 396–398, 1075–1078. [Google Scholar] [CrossRef]
- Touyama, R.; Takeda, Y.; Inoue, K.; Kawamura, I.; Yatsuzuka, M.; Ikumoto, T.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to the blue pigments. Chem. Pharm. Bull. 1994, 42, 668–673. [Google Scholar] [CrossRef]
- Minosasa, Y.; Tanaka, H. Bright-Blue Plant Pigment and its Manufacture from Gardenia. Jpn. Patent JP 01,179,690, 30 December 1989. [Google Scholar]
- Gao, L.; Deng, Q.Y.; Jiang, Y.Q.; Li, G.Y.; Zhang, Z. Research on the isolation and purification technology on gardenia yellow pigment by HPD-400 macroporous resin. Med. Plant 2012, 3, 55–58. [Google Scholar]
- Choe, J.-S. A Method for Extracting Crocin from Fructus Gardeniae. Kr. Patent KR 9,404,527, 30 March 1994. [Google Scholar]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Martinez, T. Understanding mechanochemistry from first principles. In Abstracts of Papers, 249th ACS National Meeting&Exposition, Denver, CO, USA, 22–26 March 2015; American Chemical Society: Washington, DC, USA, 2015. PMSE-103. [Google Scholar]
- Shon, I.-J.; Kang, H.-S. Mechanochemical synthesis and rapid consolidation of nanostructured CoTi-Al2O3 composite by pulsed current activated sintering and its mechanical properties. J. Nanosci. Nanotechnol. 2015, 15, 5330–5333. [Google Scholar] [CrossRef] [PubMed]
- Taubman, A.B.; Yanova, L.P.; Blyskosh, G.S. Mechanochemical grafting of polymers on filler surface as method of increasing its activity. J. Polym. Sci. Pol. Chem. 1971, 9, 27–32. [Google Scholar] [CrossRef]
- Xue, J.; Wan, D.; Lee, S.-E.; Wang, J. Mechanochemical synthesis of lead zirconate titanate from mixed oxides. J. Am. Ceram. Soc. 1999, 82, 1687–1692. [Google Scholar] [CrossRef]
- Muralidhar, L.; Girija, C.R. Simple and practical procedure for Knoevenagel condensation under solvent-free conditions. J. Saudi Chem. Soc. 2014, 18, 541–544. [Google Scholar] [CrossRef]
- Aakeroey, C.B.; Sinha, A.S.; Epa, K.N.; Spartz, C.L.; Desper, J. A versatile and green mechanochemical route for aldehyde-oxime conversions. Chem. Commun. 2012, 48, 11289–11291. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Nakai, S.; Hosomi, M. Elucidation of Degradation Mechanism of Dioxins during Mechanochemical Treatment. Environ. Sci. Technol. 2005, 39, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Peng, Z.; Lu, S.Y.; Li, X.D.; Ni, M.J.; Cen, K.F.; Dai, H.F. Degradation of PCDDS/FS by mechanochemical treatment of fly ash from medical waste incinerator. J. Hazard. Mater. 2007, 147, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Korolev, K.G.; Lomovskii, O.I.; Rozhanskaya, O.A.; Vasi’ev, V.G. Mechanochemical Preparation of Water-Soluble Forms of Triterpene Acids. Chem. Nat. Compd. 2003, 39, 366–372. [Google Scholar] [CrossRef]
- Xie, J.; Li, H.; Zhu, X.; Wang, P.; Su, W. Efficient and selective extraction of magnolol from Magnolia officinalis by mechanochemical extraction technique. Chem. Eng. Proc. Proc. Intensif. 2011, 50, 325–330. [Google Scholar] [CrossRef]
- Zhu, X.; Su, W.; Wang, P.; Li, F.; Wang, J.; Xie, J.; (Univ. Zhejiang Technology; Zhejiang Suichang Limin Pharmaceutical Co., CN). Method for Extracting Gardenia Yellow Pigment from Gardeniae Longicarpae Fruit. Chin. Patent CN 102344692 A, 10 August 2012. [Google Scholar]
- Xie, J.; Lin, Y.-S.; Shi, X.-J.; Zhu, X.-Y.; Su, W.-K.; Wang, P. Mechanochemical-assisted extraction of flavonoids from bamboo (Phyllostachys edulis) leaves. Ind. Crop. Prod. 2013, 43, 276–282. [Google Scholar] [CrossRef]
- Liao, F. Research development of gardenia. Guangzhou Huagong 2013, 41, 12–13. [Google Scholar]
- Li, X.H.; Zhao, P.; Ji, Q.R. Comparison of Different Extraction Process to The Effect about Gardenia Yellow Pigment. Food Res. Dev. 2006, 27, 192–194. [Google Scholar]
- National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2015; Volume 1, p. 248. [Google Scholar]
- Sample Availability: Not available.







| Entry | Rotation Rate (rpm) | Powder-to-Ball Weight Ratio a (g/g) | Milling Time (min) | Gardenia Yellow Extraction Rate (%) b | Geniposide Extraction Rate (%) c | ||
|---|---|---|---|---|---|---|---|
| Water | Alcohol | Water | Alcohol | ||||
| 1 | 100 | 1:5 | 5 | 0.65 ± 0.03 | 1.09 ± 0.03 | 3.06 ± 0.14 | 0.40 ± 0.02 |
| 2 | 200 | 1:5 | 5 | 0.76 ± 0.02 | 1.18 ± 0.06 | 3.46 ± 0.16 | 0.79 ± 0.03 |
| 3 | 400 | 1:5 | 5 | 0.72 ± 0.02 | 1.15 ± 0.05 | 3.38 ± 0.12 | 0.71 ± 0.03 |
| 4 | 600 | 1:5 | 5 | 0.52 ± 0.02 | 0.96 ± 0.02 | 3.04 ± 0.12 | 0.44 ± 0.02 |
| 5 | 200 | 1:1 | 5 | 0.67 ± 0.03 | 1.14 ± 0.03 | 3.04 ± 0.07 | 0.40 ±0.01 |
| 6 | 200 | 1:2 | 5 | 0.71 ± 0.01 | 1.16 ± 0.03 | 3.23 ± 0.15 | 0.57 ± 0.03 |
| 7 | 200 | 1:3 | 5 | 0.73 ± 0.03 | 1.20 ± 0.06 | 3.30 ± 0.09 | 0.65 ± 0.02 |
| 8 | 200 | 1:6 | 5 | 0.73 ± 0.03 | 1.16 ± 0.02 | 3.48 ± 0.17 | 0.80 ± 0.03 |
| 9 | 200 | 1:8 | 5 | 0.62 ± 0.03 | 1.06 ± 0.04 | 3.38 ± 0.07 | 0.71 ±0.03 |
| 10 | 200 | 1:10 | 5 | 0.44 ± 0.01 | 0.90 ± 0.04 | 3.29 ± 0.13 | 0.62 ± 0.03 |
| 11 | 200 | 1:5 | 1 | 0.73 ± 0.03 | 1.12 ± 0.04 | 2.75 ± 0.10 | 0.44 ± 0.01 |
| 12 | 200 | 1:5 | 10 | 0.77 ± 0.04 | 1.17 ± 0.03 | 3.50 ± 0.13 | 0.71 ± 0.03 |
| 13 | 200 | 1:5 | 20 | 0.76 ± 0.02 | 1.18 ± 0.05 | 3.49 ± 0.15 | 0.79 ± 0.03 |
| 14 | 200 | 1:5 | 30 | 0.77 ± 0.03 | 1.09 ± 0.04 | 3.49± 0.13 | 0.40 ± 0.01 |
| 15 | Mixture without milling | 0.098 ± 0.022 | 0.88 ± 0.02 | 0.112 ± 0.001 | 1.53 ± 0.04 | ||
| Entry | Liquid-Solid Ratio (mL/g) | Temperature (°C) | Extraction Time (min) | Geniposide Extraction Rate (%) |
|---|---|---|---|---|
| 1 | 5:1 | 20 | 5 | 2.48 ± 0.12 |
| 2 | 10:1 | 20 | 5 | 3.59 ± 0.13 |
| 3 | 20:1 | 20 | 5 | 3.50 ± 0.09 |
| 4 | 10:1 | 40 | 5 | 3.59 ± 0.18 |
| 5 | 10:1 | 60 | 5 | 3.59 ± 0.11 |
| 6 | 10:1 | 80 | 5 | 3.55 ± 0.14 |
| 7 | 10:1 | 100 | 5 | 3.57 ± 0.11 |
| 8 | 10:1 | 20 | 10 | 3.57 ± 0.08 |
| 9 | 10:1 | 20 | 20 | 3.53 ± 0.09 |
| 10 | 10:1 | 20 | 30 | 3.52 ± 0.15 |
| Entry | Liquid-Solid Ratio (mL/g) | Temperature (°C) | Extraction Time (min) | Gardenia Yellow Extraction Rate (%) c |
|---|---|---|---|---|
| 1 | 2:1 | 80 | 5 | 1.31 ± 0.04 |
| 2 | 5:1 | 80 | 5 | 1.45 ± 0.04 |
| 3 | 10:1 | 80 | 5 | 1.42 ± 0.06 |
| 4 | 20:1 | 80 | 5 | 1.36 ± 0.05 |
| 5 | 5:1 | 80 | 10 | 1.45 ± 0.05 |
| 6 | 5:1 | 80 | 20 | 1.47 ± 0.03 |
| 7 | 5:1 | 80 | 30 | 1.49 ± 0.07 |
| 8 | 5:1 | 20 | 5 | 1.13 ± 0.03 |
| 9 | 5:1 | 40 | 5 | 1.24 ± 0.05 |
| 10 | 5:1 | 60 | 5 | 1.40 ± 0.04 |
| Entry | Method | Solvent | Coler Value | OD | Qualified |
|---|---|---|---|---|---|
| 1 | Reflux | 60% alcohol | 187 | 2.72 | N |
| 2 | Enzyme | Water | 56 | 3.26 | N |
| 3 | Ultrasound | 60% alcohol | 241 | 2.54 | N |
| 4 | Mecanical chemistry | Water/60% alcohol | 150 | 0.38 ± 0.055% (Geniposide content) a | Y |
| Entry | Samples | BET Surface Area a (m2/g) | BJH Desorption Average Pore Diameter a (nm) | Micropore Volume a (cm3/g) |
|---|---|---|---|---|
| 1 | Activated carbon | 2647.96 | 4.54 | 0.39 |
| 2 | Milled activated carbon b | 1520.48 | 4.78 | 0.25 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Yu, J.; Feng, W.; Su, W. Selective Extraction of Gardenia Yellow and Geniposide from Gardenia jasminoides by Mechanochemistry. Molecules 2016, 21, 540. https://doi.org/10.3390/molecules21050540
Xu W, Yu J, Feng W, Su W. Selective Extraction of Gardenia Yellow and Geniposide from Gardenia jasminoides by Mechanochemistry. Molecules. 2016; 21(5):540. https://doi.org/10.3390/molecules21050540
Chicago/Turabian StyleXu, Wenhao, Jingbo Yu, Wen Feng, and Weike Su. 2016. "Selective Extraction of Gardenia Yellow and Geniposide from Gardenia jasminoides by Mechanochemistry" Molecules 21, no. 5: 540. https://doi.org/10.3390/molecules21050540