Recent Advance in Co3O4 and Co3O4-Containing Electrode Materials for High-Performance Supercapacitors
Abstract
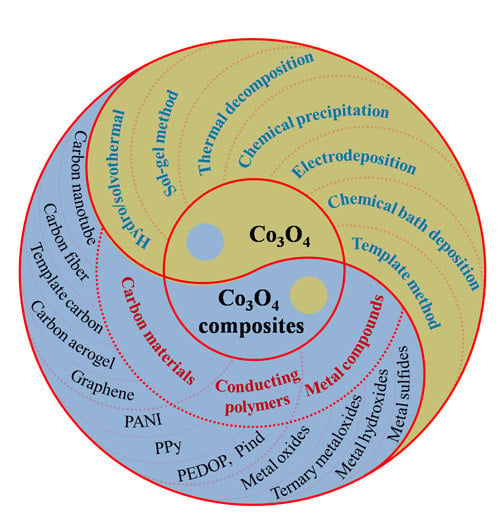
:1. Introduction
2. Synthesis and Performance of Co3O4
2.1. Hydrothermal or Solvothermal Method
2.2. Sol–Gel Method
2.3. Thermal Decomposition
2.4. Chemical Precipitation
2.5. Electrodeposition
2.6. Chemical Bath Deposition
2.7. Template Method
2.8. Performance Statistics of Different Co3O4 Materials
3. Advance of Co3O4-Containing Composites
3.1. Co3O4/Carbon Composites
3.1.1. Co3O4/Carbon Nanotube (CNT) Composites
3.1.2. Co3O4/Carbon Fiber (CF) Composites
3.1.3. Co3O4/Template Carbon (TC) Composites
3.1.4. Co3O4/Carbon Aerogel (CA) Composites
3.1.5. Co3O4/Graphene Composites
3.2. Co3O4/Conductive Polymer Composites
3.2.1. Co3O4/PANI Composites
3.2.2. Co3O4/PPy Composites
3.2.3. Co3O4/Other Conductive Polymer Composites
3.3. Co3O4/Metal Compound Composites
3.3.1. Co3O4/Metal Oxide Composites
3.3.2. Co3O4/Ternary Metal Oxide Composites
3.3.3. Co3O4/Metal Hydroxide Composites

3.3.4. Co3O4/Metal Sulfide Composites
3.4. Co3O4/Multiple Materials Composites
3.5. Performance Comparison of Co3O4-Containing Composites
4. Conclusions and Perspective
4.1. Conclusions
4.2. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, M.M.; Zhao, Q.X.; Li, B.; Xue, H.G.; Pang, H.; Chen, C.Y. Recent progress in layered double hydroxide based materials for electrochemical capacitors: Design, synthesis and performance. Nanoscale 2017, 9, 15206–15225. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [Green Version]
- Kasnatscheew, J.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Interfaces and Materials in Lithium Ion Batteries: Challenges for Theoretical Electrochemistry. In Modeling Electrochemical Energy Storage at the Atomic Scale; Springer: Münster, Germany, 2018; pp. 23–51. [Google Scholar]
- Miller, J.R. Reliability of Electrochemical Capacitors. In Supercapacitors: Materials, Systems, and Applications; Wiley-VCH: Weinheim, Germany, 2013; Chapter 13; pp. 473–507. [Google Scholar]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.W. Mesoporous Transition Metal Oxides for Supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Ujjain, S.K.; Singh, G.; Sharma, R.K. Co3O4@Reduced Graphene Oxide Nanoribbon for high performance Asymmetric Supercapacitor. Electrochim. Acta 2015, 169, 276–282. [Google Scholar] [CrossRef]
- Guan, Q.; Cheng, J.; Wang, B.; Ni, W.; Gu, G.; Li, X.; Huang, L.; Yang, G.; Nie, F. Needle-like Co3O4 Anchored on the Graphene with Enhanced Electrochemical Performance for Aqueous Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7626–7632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, W.; Song, S.; Hao, Q.; Fan, W.; Xia, X.; Zhang, X.; Wang, Q.; liu, C.; Yan, D. Solvothermal synthesis and electrochemical performance in super-capacitors of Co3O4/C flower-like nanostructures. J. Power Sources 2014, 248, 1281–1289. [Google Scholar] [CrossRef]
- Liu, X.; Long, Q.; Jiang, C.; Zhan, B.; Li, C.; Liu, S.; Zhao, Q.; Huang, W.; Dong, X. Facile and green synthesis of mesoporous Co3O4 nanocubes and their applications for supercapacitors. Nanoscale 2013, 5, 6525–6529. [Google Scholar] [CrossRef]
- Xiang, C.; Li, M.; Zhi, M.; Manivannan, A.; Wu, N. A reduced graphene oxide/Co3O4 composite for supercapacitor electrode. J. Power Sources 2013, 226, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhu, J.; Bi, H.; Meng, X.; Liang, S.; Zhang, L.; Wang, X. Graphene-based 3D composite hydrogel by anchoring Co3O4 nanoparticles with enhanced electrochemical properties. Phys. Chem. Chem. Phys. 2013, 15, 12940–12945. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ma, M.Z.; Yang, J.; Sun, C.C.; Su, H.Q.; Huang, W.; Dong, X.C. Shape-controlled synthesis of NiCo2S4 and their charge storage characteristics in supercapacitors. Nanoscale 2014, 6, 9824–9830. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Gu, C.; Ma, Y. Electropolymerized Conjugated Microporous Poly(zinc-porphyrin) Films as Potential Electrode Materials in Supercapacitors. Adv. Energy. Mater 2015, 5, 1402175. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Li, X.; Xu, D.; Wang, Z.; Guo, H.; Zhang, K. Three-dimensional hierarchical Co3O4/CuO nanowire heterostructure arrays on nickel foam for high-performance lithium ion batteries. Nano Energy 2014, 6, 19–26. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, L.; Xiao, Y.; Shen, L.; Chen, Q.; Shi, W. Hydrogenated CoOx nanowire@Ni(OH)2 nanosheet core-shell nanostructures for high-performance asymmetric supercapacitors. Nanoscale 2014, 6, 6772–6781. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Gu, C.; Yao, M.; Yang, B.; Lu, P.; Ma, Y. High performance, flexible, poly(3,4-ethylenedioxythiophene) supercapacitors achieved by doping redox mediators in organogel electrolytes. J. Power Sources 2016, 332, 413–419. [Google Scholar] [CrossRef]
- Xiong, W.; Pan, X.; Li, Y.; Chen, X.; Zhu, Y.; Yang, M.; Zhang, Y. Hierarchical Co3O4@PPy core/shell nanowire arrays on nickel foam for electrochemical energy storage. Mater. Lett. 2015, 157, 23–26. [Google Scholar] [CrossRef]
- Xu, H.; Hai, Z.; Diwu, J.; Zhang, Q.; Gao, L.; Cui, D.; Zang, J.; Liu, J.; Xue, C. Synthesis and microwave absorption properties of core-shell structured Co3O4-PANI nanocomposites. J. Nanomater. 2015, 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Lu, Y.; Li, S.; Wang, X.; Wang, X.; Liang, J. Carbon foam@reduced graphene oxide scaffold grown with polyaniline nanofibers for high performance symmetric supercapacitor. Electrochim. Acta 2019, 294, 376–382. [Google Scholar] [CrossRef]
- Tang, C.H.; Yin, X.; Gong, H. Superior performance asymmetric supercapacitors based on a directly grown commercial mass 3D Co3O4@Ni(OH)2 core-shell electrode. ACS Appl. Mater. Interfaces 2013, 5, 10574–10582. [Google Scholar] [CrossRef]
- Deng, J.; Kang, L.; Bai, G.; Li, Y.; Li, P.; Liu, X.; Yang, Y.; Gao, F.; Liang, W. Solution combustion synthesis of cobalt oxides (Co3O4 and Co3O4/CoO) nanoparticles as supercapacitor electrode materials. Electrochim. Acta 2014, 132, 127–135. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Zhang, L.; Wen, Z.; Liu, Q. Facile synthesis of hierarchical Co3O4@MnO2 core–shell arrays on Ni foam for asymmetric supercapacitors. J. Power Sources 2014, 252, 98–106. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, Y.; Wang, Z.; Fu, H.; Zhang, X.; Chen, Y.; Zhang, H.; Li, X. Unveiling the dynamic capacitive storage mechanism of Co3O4 @NiCo2O4 hybrid nanoelectrodes for supercapacitor applications. Electrochim. Acta 2014, 145, 177–184. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, B.; Liu, R.; Wang, Q.F.; Hou, X.J.; Chen, D.; Wang, R.M.; Shen, G.Z. Fiber-Based Flexible All-Solid-State Asymmetric Supercapacitors for Integrated Photodetecting System. Angew. Chem. Int. Ed. 2014, 53, 1849–1853. [Google Scholar] [CrossRef]
- Kong, D.Z.; Luo, J.S.; Wang, Y.L.; Ren, W.N.; Yu, T.; Luo, Y.S.; Yang, Y.P.; Cheng, C.W. Three-Dimensional Co3O4@MnO2 Hierarchical Nanoneedle Arrays: Morphology Control and Electrochemical Energy Storage. Adv. Funct. Mater 2014, 24, 3815–3826. [Google Scholar] [CrossRef]
- Yi, C.Q.; Zou, J.P.; Yang, H.Z.; Leng, X. A facile hydrothermal synthesis of graphene/RuO2/Co3O4 nanocomposites with high pseudocapacity. New J. Chem. 2018, 42, 7066–7072. [Google Scholar] [CrossRef]
- Gong, X.F.; Cheng, J.P.; Liu, F.; Zhang, L.; Zhang, X.B. Nickel-Cobalt hydroxide microspheres electrodepositioned on nickel cobaltite nanowires grown on Ni foam for high-performance pseudocapacitors. J. Power Sources 2014, 267, 610–616. [Google Scholar] [CrossRef]
- Guo, J.X.; Chen, L.; Zhang, X.; Jiang, B.; Ma, L.Z. Sol–gel synthesis of mesoporous Co3O4 octahedra toward high-performance anodes for lithium-ion batteries. Electrochim. Acta 2014, 129, 410–415. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, A.; Zhu, Q.; Nie, Z.; Zhang, Y.; Tang, Y.; Liang, S.; Cao, G. Facile synthesis of nanorod-assembled multi-shelled Co3O4 hollow microspheres for high-performance supercapacitors. J. Power Sources 2014, 272, 107–112. [Google Scholar] [CrossRef]
- Lee, M.; Wee, B.H.; Hong, J.D. High performance flexible supercapacitor electrodes composed of ultralarge graphene sheets and vanadium dioxide. Adv. Energy Mater. 2015, 5, 1401890. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-Based Nanocomposites for Energy Storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar]
- Yuan, K.; Xu, Y.; Uihlein, J.; Brunklaus, G.; Shi, L.; Heiderhoff, R.; Que, M.; Forster, M.; Chassé, T.; Pichler, T. Straightforward generation of pillared, microporous graphene frameworks for use in supercapacitors. Adv. Mater. 2015, 27, 6714–6721. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, C.; Chang, J.; Chen, L.; Wu, D.; Xu, F.; Jiang, K. Balanced energy density and power density: Asymmetric supercapacitor based on activated fullerene carbon soot anode and graphene-Co3O4 composite cathode. Electrochim. Acta 2018, 260, 932–943. [Google Scholar] [CrossRef]
- Qiu, K.; Lu, Y.; Cheng, J.; Yan, H.; Hou, X.; Zhang, D.; Lu, M.; Liu, X.; Luo, Y. Ultrathin mesoporous Co3O4 nanosheets on Ni foam for high-performance supercapacitors. Electrochim. Acta 2015, 157, 62–68. [Google Scholar] [CrossRef]
- Venkatachalam, V.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Shape controlled synthesis of rod-like Co3O4 nanostructures as high-performance electrodes for supercapacitor applications. J. Mater. Sci. Mater. El. 2018, 29, 6059–6067. [Google Scholar] [CrossRef]
- Wang, X.; Xia, H.; Wang, X.; Gao, J.; Shi, B.; Fang, Y. Facile synthesis ultrathin mesoporous Co3O4 nanosheets for high-energy asymmetric supercapacitor. J. Alloys Compd. 2016, 686, 969–975. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, B.; Su, H.; Zhang, H.; Zhang, L.; Yang, W. Controllable synthesis of self-assembly Co3O4 nanoflake microspheres for electrochemical performance. Nanotechnology 2016, 27, 355603. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, L.; Zhang, H.; Zhang, Q.; Chen, W.; Zhu, J.; Song, D. Two-dimensional Co3O4 thin sheets assembled by 3D interconnected nanoflake array framework structures with enhanced supercapacitor performance derived from coordination complexes. Chem. Eng. J. 2016, 292, 1–12. [Google Scholar] [CrossRef]
- Deori, K.; Ujjain, S.K.; Sharma, R.K.; Deka, S. Morphology controlled synthesis of nanoporous Co3O4 nanostructures and their charge storage characteristics in supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10665–10672. [Google Scholar] [CrossRef]
- Meng, T.; Xu, Q.Q.; Wang, Z.H.; Li, Y.T.; Gao, Z.M.; Xing, X.Y.; Ren, T.Z. Co3O4 Nanorods with Self-assembled Nanoparticles in Queue for Supercapacitor. Electrochim. Acta 2015, 180, 104–111. [Google Scholar] [CrossRef]
- Liu, F.; Su, H.; Jin, L.; Zhang, H.; Chu, X.; Yang, W. Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 2017, 505, 796–804. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Zhou, W.W.; Wan, S.S.; Du, W.; Zhang, H.L.; Ding, C.Y.; Du, Y.; Zhu, L.J. Facile synthesis of homogeneous core-shell Co3O4 mesoporous nanospheres as high performance electrode materials for supercapacitor. J. Alloys Compd. 2019, 774, 137–144. [Google Scholar] [CrossRef]
- George, G.; Elias, L.; Hegde, A.C.; Anandhan, S. Morphological and structural characterisation of sol–gel electrospun Co3O4 nanofibres and their electro-catalytic behaviour. RSC Adv. 2015, 5, 40940–40949. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Sol–gel synthesis, characterisation, and photocatalytic activity of porous spinel Co3O4 nanosheets. Chem. Pap. 2014, 68, 1087–1096. [Google Scholar] [CrossRef]
- Lima-Tenorio, M.K.; Ferreira, C.S.; Rebelo, Q.H.F.; de Souza, R.F.B.; Passos, R.R.; Pineda, E.A.G.; Pocrifka, L.A. Pseudocapacitance Properties of Co3O4 Nanoparticles Synthesized Using a Modified Sol–Gel Method. Mater. Res 2018, 21, e20170521. [Google Scholar] [CrossRef]
- Lakehal, A.; Bedhiaf, B.; Bouaza, A.; Benhebal, H.; Ammari, A.; Dalache, C. Structural, optical and electrical properties of Ni-doped Co3O4 prepared via Sol–Gel technique. Mater. Res 2018, 21, e20170545. [Google Scholar] [CrossRef]
- Peterson, G.R.; Hung-Low, F.; Gumeci, C.; Bassett, W.P.; Korzeniewski, C.; Hope-Weeks, L.J. Preparation–Morphology–Performance Relationships in Cobalt Aerogels as Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 1796–1803. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M.; Nadri, G. Characterization of Cobalt Oxide Nanoparticles Prepared by the Thermal Decomposition of Co(NH3)5(H2O)(NO3)3 Complex and Study of Their Photocatalytic Activity. Acta Chim. Slov. 2016, 63, 335–343. [Google Scholar] [CrossRef]
- Xu, Y.N.; Ding, Q.; Li, L.; Xie, Z.J.; Jiang, G.X. Facile fabrication of porous Co3O4 nanowires for high performance supercapacitors. New J. Chem. 2018, 42, 20069–20073. [Google Scholar] [CrossRef]
- Kong, S.Y.; Yang, F.; Cheng, K.; Ouyang, T.; Ye, K.; Wang, G.L.; Cao, D.X. In-situ growth of cobalt oxide nanoflakes from cobalt nanosheet on nickel foam for battery-type supercapacitors with high specific capacity. J. Electroanal. Chem. 2017, 785, 103–108. [Google Scholar] [CrossRef]
- Lv, Y.N.; Dong, G.X.; Li, L.; Kang, J.R.; Han, W.D. Cobalt-Nickel Oxides with Three-Dimensional Hexagon Films for High Performance Supercapacitors. Nano 2018, 13, 1850032. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, Q.; Wang, T. Morphology-controllable synthesis of cobalt oxalates and their conversion to mesoporous Co3O4 nanostructures for application in supercapacitors. Inorg. Chem. 2011, 50, 6482–6492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-Nanonet Hollow Structured Co3O4 for High Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Song, F.M.; Zan, G.T.; Chen, Y.; Wu, Q.S.; Xu, Y.Y. In situ transformation of iron-group ternary metal oxides nanocubes from Co/Ni-PBA for high-performance supercapacitors. J. Alloys Compd. 2018, 741, 633–641. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for High-Performance Supercapacitor Applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Ren, S.R.; Guo, Y.K.; Ju, L.L.; Xiao, H.; Hu, A.M.; Li, M. Facile synthesis of petal-like nanocrystalline Co3O4 film using direct high-temperature oxidation. J. Mater. Sci. 2019, 54, 7922–7930. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ahmadi, R.; Gharailou, D.; Ganjali, M.R.; Norouzi, P. A facile route to preparation of Co3O4 nanoplates and investigation of their charge storage ability as electrode material for supercapacitors. J. Mater. Sci. Mater. El. 2016, 27, 8623–8632. [Google Scholar] [CrossRef]
- Guo, X.G.; Li, X.M.; Xiong, Z.S.; Lai, C.; Li, Y.; Huang, X.Y.; Bao, H.B.; Yin, Y.J.; Zhu, Y.H.; Zhang, D.X. A comprehensive investigation on electrophoretic self-assembled nano-Co3O4 films in aqueous solution as electrode materials for supercapacitors. J. Nanopart. Res. 2016, 18, 144. [Google Scholar] [CrossRef]
- Pan, X.; Chen, X.; Li, Y.; Yu, Z. Facile Synthesis of Co3O4 Nanosheets Electrode with Ultrahigh Specific Capacitance for Electrochemical Supercapacitors. Electrochim. Acta 2015, 182, 1101–1106. [Google Scholar] [CrossRef]
- Pan, G.X.; Xia, X.H.; Cao, E.; Chen, J.; Zhang, Y.J. Template-free synthesis of hierarchical porous Co3O4 microspheres and their application for electrochemical energy storage. Electrochim. Acta 2015, 173, 385–392. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kulkarni, S.B. Chemical synthesis of Co3O4 nanowires for symmetric supercapacitor device. J. Mater. Sci. Mater. El. 2018, 29, 16401–16409. [Google Scholar] [CrossRef]
- Chen, M.H.; Ge, Q.X.; Qi, M.L.; Liang, X.Q.; Wang, F.; Chen, Q.G. Cobalt oxides nanorods arrays as advanced electrode for high performance supercapacitor. Surf. Coat. Technol. 2019, 360, 73–77. [Google Scholar] [CrossRef]
- Yao, M.; Hu, Z.; Xu, Z.; Liu, Y. Template synthesis of 1D hierarchical hollow Co3O4 nanotubes as high performance supercapacitor materials. J. Alloys Compd. 2015, 644, 721–728. [Google Scholar] [CrossRef]
- Li, G.; Hua, X.; Liu, P.; Xie, Y.; Han, L. Porous Co3O4 microflowers prepared by thermolysis of metal-organic framework for supercapacitor. Mater. Chem. Phys. 2015, 168, 127–131. [Google Scholar] [CrossRef]
- Xiao, Z.; Fan, L.; Xu, B.; Zhang, S.; Kang, W.; Kang, Z.; Lin, H.; Liu, X.; Zhang, S.; Sun, D. Green Fabrication of Ultrathin Co3O4 Nanosheets from Metal–Organic Framework for Robust High-Rate Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 41827–41836. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Zhou, Z.; Zhao, X.; Zhang, W.; An, C. Ultrathin Metal–Organic Framework Nanosheet-Derived Ultrathin Co3O4 Nanomeshes with Robust Oxygen-Evolving Performance and Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 23721–23730. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, C.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. MOF-templated syntheses of porous Co3O4 hollow spheres and micro-flowers for enhanced performance in supercapacitors. CrystEngComm 2018, 20, 3812–3816. [Google Scholar] [CrossRef]
- Yadav, A.A.; Chavan, U.J. Electrochemical supercapacitive performance of spray deposited Co3O4 thin film nanostructures. Electrochim. Acta 2017, 232, 370–376. [Google Scholar] [CrossRef]
- Duan, Y.; Hu, T.; Yang, L.; Gao, J.; Guo, S.; Hou, M.; Ye, X. Facile fabrication of electroactive microporous Co3O4 through microwave plasma etching for supercapacitors. J. Alloys Compd. 2019, 771, 156–161. [Google Scholar] [CrossRef]
- Kumar, M.; Subramania, A.; Balakrishnan, K. Preparation of electrospun Co3O4 nanofibers as electrode material for high performance asymmetric supercapacitors. Electrochim. Acta 2014, 149, 152–158. [Google Scholar] [CrossRef]
- You, Y.; Zheng, M.; Ma, L.; Yuan, X.; Zhang, B.; Li, Q.; Wang, F.; Song, J.; Jiang, D.; Liu, P.; et al. Galvanic displacement assembly of ultrathin Co3O4 nanosheet arrays on nickel foam for a high-performance supercapacitor. Nanotechnology 2017, 28, 105604. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, Y.Q.; Yang, G.W. A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 2016, 8, 4227–4235. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, S.; Bai, Z.; Dai, P.; Yu, X.; Wu, M.; Hu, H. Facile fabrication and configuration design of Co3O4 porous acicular nanorod arrays on Ni foam for supercapacitors. Nanotechnology 2018, 29, 315402. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Qian, X.; Wang, X.H.; Cao, Y.Q.; Zhang, Q.; Li, A.D.; Wang, J. Atomic layer deposition of Co3O4 on carbon nanotubes/carbon cloth for high-capacitance and ultrastable supercapacitor electrode. Nanotechnology 2015, 26, 094001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, M.; Li, T.; Lu, T.; Pan, L. In situ synthesis of porous Co3O4 polyhedra/carbon nanotubes heterostructures for highly efficient supercapacitors. Ionics 2017, 23, 2175–2183. [Google Scholar] [CrossRef]
- Kazazi, M.; Sedighi, A.R.; Mokhtari, M.A. Pseudocapacitive performance of electrodeposited porous Co3O4 film on electrophoretically modified graphite electrodes with carbon nanotubes. Appl. Surf. Sci. 2018, 441, 251–257. [Google Scholar] [CrossRef]
- Durukan, M.B.; Yuksel, R.; Unalan, H.E. Cobalt Oxide Nanoflakes on Single Walled Carbon Nanotube Thin Films for Supercapacitor Electrodes. Electrochim. Acta 2016, 222, 1475–1482. [Google Scholar] [CrossRef]
- Zou, Y.; Cai, C.; Xiang, C.; Huang, P.; Chu, H.; She, Z.; Xu, F.; Sun, L.; Kraatz, H.-B. Simple synthesis of core-shell structure of Co–Co3O4@carbon-nanotube-incorporated nitrogen-doped carbon for high-performance supercapacitor. Electrochim. Acta 2018, 261, 537–547. [Google Scholar] [CrossRef]
- Ramesh, S.; Haldorai, Y.; Sivasamy, A.; Kim, H.S. Nanostructured Co3O4/nitrogen doped carbon nanotube composites for high-performance supercapacitors. Mater. Lett. 2017, 206, 39–43. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, C. Amorphous FeOOH nanorods and Co3O4 nanoflakes as binder-free electrodes for high-performance all-solid-state asymmetric supercapacitors. CrystEngComm 2019, 21, 662–672. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite nanofibers as advanced materials for Li-ion, Li-O2 and Li-S batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef] [Green Version]
- Abouali, S.; Garakani, M.A.; Zhang, B.; Xu, Z.L.; Heidari, E.K.; Huang, J.Q.; Huang, J.; Kim, J.K. Electrospun Carbon Nanofibers with in Situ Encapsulated Co(3)O(4) Nanoparticles as Electrodes for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 13503–13511. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Man, P.; Zhang, Q.; He, B.; Zhou, Z.; Li, C.; Wang, X.; Guo, J.; Zhao, J.; Xie, L.; et al. Hierarchically-structured Co3O4 nanowire arrays grown on carbon nanotube fibers as novel cathodes for high-performance wearable fiber-shaped asymmetric supercapacitors. Appl. Surf. Sci. 2018, 447, 795–801. [Google Scholar] [CrossRef]
- Shi, Z.; Xing, L.; Liu, Y.; Gao, Y.; Liu, J. A porous biomass-based sandwich-structured Co3O4@Carbon Fiber@Co3O4 composite for high-performance supercapacitors. Carbon 2018, 129, 819–825. [Google Scholar] [CrossRef]
- Chen, M.H.; Chen, S.; Qi, M.L.; Zhang, J.W.; Yin, J.H.; Chen, Q.G.; Xia, X.H. Carbon cloth/cobalt oxide integrated electrode as flexible cathode of alkaline batteries. Mater. Technol. 2016, 31, 492–496. [Google Scholar] [CrossRef]
- Aldama, I.; Barranco, V.; Centeno, T.A.; Ibanez, J.; Rojo, J.M. Composite Electrodes Made from Carbon Cloth as Supercapacitor Material and Manganese and Cobalt Oxide as Battery One. J. Electrochem. Soc. 2016, 163, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, S.; Kamatchi Kamaraj, P. Fabrication of Natural Polymer Assisted Mesoporous Co3O4/Carbon Composites for Supercapacitors. Electrochim. Acta 2015, 168, 50–58. [Google Scholar] [CrossRef]
- Zhou, S.S.; Ye, Z.C.; Hu, S.Z.; Hao, C.; Wang, X.H.; Huang, C.X.; Wu, F.S. Designed formation of Co3O4/ZnCo2O4/CuO hollow polyhedral nanocages derived from zeolitic imidazolate framework-67 for high-performance supercapacitors. Nanoscale 2018, 10, 15771–15781. [Google Scholar] [CrossRef]
- Hao, S.J.; Zhang, B.W.; Wang, Y.; Li, C.J.; Feng, J.Y.; Ball, S.; Srinivasan, M.; Wu, J.S.; Huang, Y.Z. Hierarchical three-dimensional Fe3O4@porous carbon matrix/graphene anodes for high performance lithium ion batteries. Electrochim. Acta 2018, 260, 965–973. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Liu, Q.L.; Gu, J.J.; Zhang, W.; Zhang, D. A facile low-temperature synthesis of highly distributed and size-tunable cobalt oxide nanoparticles anchored on activated carbon for supercapacitors. J. Power Sources 2015, 273, 945–953. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Q.; Kang, D.; Gu, J.; Zhang, W.; Zhang, D. Facile Self-Cross-Linking Synthesis of 3D Nanoporous Co3O4/Carbon Hybrid Electrode Materials for Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 16035–16044. [Google Scholar] [CrossRef]
- Xu, Z.M.; Younis, A.; Chu, D.W.; Ao, Z.M.; Xu, H.L.; Li, S.A. Electrodeposition of Mesoporous Co3O4 Nanosheets on Carbon Foam for High Performance Supercapacitors. J. Nanomater. 2014, 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, X.; Dong, Y.; Wang, L.; Jin, C.; Zhou, N.; Chen, M.; Dong, Y.; Xie, Z.; Zhang, C. Porous cobalt oxides/carbon foam hybrid materials for high supercapacitive performance. J. Colloid Interface Sci. 2019, 542, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, I.; Ju, H.; Kim, J. Introduction of Co3O4 into activated honeycomb-like carbon for the fabrication of high performance electrode materials for supercapacitors. Phys. Chem. Chem. Phys. 2016, 18, 9124–9132. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Choe, S.R.; Huh, Y.S.; Han, Y.K. Metal-organic framework derived nanoporous carbon/Co3O4 composite electrode as a sensing platform for the determination of glucose and high-performance supercapacitor. Carbon 2018, 127, 366–373. [Google Scholar] [CrossRef]
- Zhang, G.X.; Chen, Y.M.; Jiang, Y.J.; Lin, C.; Chen, Y.G.; Guo, H.B. Formation of CMK-3/Co3O4 nanosheets on nickel foam with markedly enhanced pseudocapacitive properties. J. Mater. Sci. Technol. 2018, 34, 1538–1543. [Google Scholar] [CrossRef]
- Hu, P.D.; Long, M.C.; Bai, X.; Wang, C.; Cai, C.Y.; Fu, J.J.; Zhou, B.X.; Zhou, Y.F. Monolithic cobalt-doped carbon aerogel for efficient catalytic activation of peroxymonosulfate in water. J. Hazard. Mater. 2017, 332, 195–204. [Google Scholar] [CrossRef]
- Esfahani, M.Z.; Aghaei, A.; Khosravi, M.; Bagheri, N.; Khakpoura, Z.; Javaheri, M. Pore structure improvement of carbon aerogel and investigation of the supercapacitive behavior of a Co3O4 nanoball/carbon aerogel composite. New J. Chem. 2017, 41, 11731–11741. [Google Scholar] [CrossRef]
- Sun, G.L.; Ma, L.Y.; Ran, J.B.; Shen, X.Y.; Tong, H. Incorporation of homogeneous Co3O4 into a nitrogen-doped carbon aerogel via a facile in situ synthesis method: Implications for high performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 9542–9554. [Google Scholar] [CrossRef]
- Cui, J.F.; Xi, Y.L.; Chen, S.; Li, D.H.; She, X.L.; Sun, J.; Han, W.; Yang, D.J.; Guo, S.J. Prolifera-Green-Tide as Sustainable Source for Carbonaceous Aerogels with Hierarchical Pore to Achieve Multiple Energy Storage. Adv. Funct. Mater. 2016, 26, 8487–8495. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.Y.; Ma, Y.B.; Yao, J.R.; Li, X.; Sun, Y.Y.; Xiong, Z.Y.; Li, D. RF magnetron sputtering synthesis of three-dimensional graphene@Co3O4 nanowire array grown on Ni foam for application in supercapacitors. J. Alloys Compd. 2018, 740, 174–179. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Hao, Y.; Yang, X.; Goddard, W.A., 3rd; Zhang, X.L.; Cao, B. Oxygen-Vacancy Abundant Ultrafine Co3O4/Graphene Composites for High-Rate Supercapacitor Electrodes. Adv. Sci. 2018, 5, 1700659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Sun, X.; Jiang, Z.; Jiang, Z.-J.; Hao, X.; Shao, D.; Maiyalagan, T. Exploration of the Active Center Structure of Nitrogen-Doped Graphene for Control over the Growth of Co3O4 for a High-Performance Supercapacitor. ACS Appl. Energy Mater. 2018, 1, 143–153. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, C.; Feng, G.; Ke, Q.; Huang, X.; Wang, J. Flexible Asymmetric Supercapacitor Based on Structure-Optimized Mn3O4/Reduced Graphene Oxide Nanohybrid Paper with High Energy and Power Density. Adv. Funct. Mater. 2015, 25, 7291–7299. [Google Scholar] [CrossRef]
- Tan, H.Y.; Yu, B.Z.; Cao, L.L.; Cheng, T.; Zheng, X.L.; Li, X.H.; Li, W.L.; Ren, Z.Y. Layer-dependent growth of two-dimensional Co3O4 nanostructure arrays on graphene for high performance supercapacitors. J. Alloys Compd. 2017, 696, 1180–1188. [Google Scholar] [CrossRef]
- Yin, D.; Huang, G.; Sun, Q.; Li, Q.; Wang, X.; Yuan, D.; Wang, C.; Wang, L. RGO/Co3O4 Composites Prepared Using GO-MOFs as Precursor for Advanced Lithium-ion Batteries and Supercapacitors Electrodes. Electrochim. Acta 2016, 215, 410–419. [Google Scholar] [CrossRef]
- Lai, C.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. High-performance double ion-buffering reservoirs of asymmetric supercapacitors based on flower-like Co3O4-G>N-PEGm microspheres and 3D rGO-CNT>N-PEGm aerogels. Nanoscale 2018, 10, 17293–17303. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on Carbon/Polyaniline Hybrids: Design and Synthesis for Supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xiao, T.; Tan, X.; Xiang, P.; Jiang, L.; Wu, D.; Li, J.; Wang, S. A new asymmetric aqueous supercapacitor: Co3O4//Co3O4@polypyrrole. J. Alloys Compd. 2017, 706, 351–357. [Google Scholar] [CrossRef]
- Qi, M.; Xie, D.; Zhong, Y.; Chen, M.; Xia, X. Smart construction of polyaniline shell on cobalt oxides as integrated core-shell arrays for enhanced lithium ion batteries. Electrochim. Acta 2017, 247, 701–707. [Google Scholar] [CrossRef]
- Mai, Y.J.; Xia, X.H.; Jie, X.H. Conformal construction of polyaniline shell on cobalt oxide nanoflake core for enhanced Li ion storage. Mater. Res. Bull. 2017, 94, 216–221. [Google Scholar] [CrossRef]
- Hai, Z.; Gao, L.; Zhang, Q.; Xu, H.; Cui, D.; Zhang, Z.; Tsoukalas, D.; Tang, J.; Yan, S.; Xue, C. Facile synthesis of core–shell structured PANI-Co3O4 nanocomposites with superior electrochemical performance in supercapacitors. Appl. Surf. Sci. 2016, 361, 57–62. [Google Scholar] [CrossRef]
- Ren, X.; Fan, H.; Ma, J.; Wang, C.; Zhang, M.; Zhao, N. Hierarchical Co3O4/PANI hollow nanocages: Synthesis and application for electrode materials of supercapacitors. Appl. Surf. Sci. 2018, 441, 194–203. [Google Scholar] [CrossRef]
- Padwal, P.M.; Kadam, S.L.; Mane, S.M.; Kulkarni, S.B. Synthesis and characterization of supercapacitive behavior of electrodeposited PANI/Co3O4 layered composite electrode. J. Chin. Chem. Soc. Taip. 2016, 4, 13–23. [Google Scholar]
- Yang, X.; Xu, K.; Zou, R.; Hu, J. A Hybrid Electrode of Co3O4@PPy Core/Shell Nanosheet Arrays for High-Performance Supercapacitors. Nanomicro. Lett. 2016, 8, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, M.; Chen, Z.; Liu, X. Hierarchical Co3O4@PPy core-shell composite nanowires for supercapacitors with enhanced electrochemical performance. Mater. Res. Bull. 2017, 96, 463–470. [Google Scholar] [CrossRef]
- Wu, X.; Meng, L.; Wang, Q.; Zhang, W.; Wang, Y. A flexible asymmetric fibered-supercapacitor based on unique Co3O4@PPy core-shell nanorod arrays electrode. Chem. Eng. J. 2017, 327, 193–201. [Google Scholar] [CrossRef]
- Ma, L.T.; Fan, H.Q.; Wei, X.Y.; Chen, S.M.; Hu, Q.Z.; Liu, Y.; Zhi, C.Y.; Lu, W.; Zapien, J.A.; Huang, H.T. Towards high areal capacitance, rate capability, and tailorable supercapacitors: Co3O4@polypyrrole core-shell nanorod bundle array electrodes. J. Mater. Chem. A 2018, 6, 19058–19065. [Google Scholar] [CrossRef]
- Ozkazanc, E. PTh/Co3O4 nanocomposites as new conducting materials for micro/nano-sized electronic devices. Polym. Eng. Sci. 2017, 57, 1168–1178. [Google Scholar]
- Reddy, B.N.; Deshagani, S.; Deepa, M.; Ghosal, P. Effective pseudocapacitive charge storage/release by hybrids of poly(3,4-ethylenedioxypyrrole) with Fe3O4 nanostructures or Co3O4 nanorods. Chem. Eng. J. 2018, 334, 1328–1340. [Google Scholar] [CrossRef]
- Raj, R.P.; Ragupathy, P.; Mohan, S. Remarkable capacitive behavior of a Co3O4–polyindole composite as electrode material for supercapacitor applications. J. Mater. Chem. A 2015, 3, 24338–24348. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y.; Wang, D.; Xu, F. Ethanol-assisted solvothermal synthesis of porous nanostructured cobalt oxides (CoO/Co3O4) for high-performance supercapacitors. Chem. Eng. J. 2015, 280, 377–384. [Google Scholar] [CrossRef]
- Hu, Q.; Gu, Z.; Zheng, X.; Zhang, X. Three-dimensional Co3O4@NiO hierarchical nanowire arrays for solid-state symmetric supercapacitor with enhanced electrochemical performances. Chem. Eng. J. 2016, 304, 223–231. [Google Scholar] [CrossRef]
- Ambare, R.C.; Bharadwaj, S.R.; Lokhande, B.J. Non-aqueous route spray pyrolyzed Ru:Co3O4 thin electrodes for supercapacitor application. Appl. Surf. Sci. 2015, 349, 887–896. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, G.; Du, Q.; Su, L.; Ma, Z.; Qin, X.; Shao, G. Co3O4@MnO2 core shell arrays on nickel foam with excellent electrochemical performance for aqueous asymmetric supercapacitor. Ionics 2017, 23, 1637–1643. [Google Scholar] [CrossRef]
- Wang, K.; Shi, Z.; Wang, Y.; Ye, Z.; Xia, H.; Liu, G.; Qiao, G. Co3O4 nanowires@MnO2 nanolayer or nanoflakes core–shell arrays for high-performance supercapacitors: The influence of morphology on performance. J. Alloys Compd. 2015, 624, 85–93. [Google Scholar] [CrossRef]
- Xing, L.; Dong, Y.; Hu, F.; Wu, X.; Umar, A. Co3O4 nanowire@NiO nanosheet arrays for high performance asymmetric supercapacitors. Dalton T. 2018, 47, 5687–5694. [Google Scholar] [CrossRef]
- Yang, F.; Xu, K.; Hu, J. Construction of Co3O4@Fe2O3 core-shell nanowire arrays electrode for supercapacitors. J. Alloys Compd. 2017, 729, 1172–1176. [Google Scholar] [CrossRef]
- Chandra Sekhar, S.; Nagaraju, G.; Yu, J.S. High-performance pouch-type hybrid supercapacitor based on hierarchical NiO-Co3O4-NiO composite nanoarchitectures as an advanced electrode material. Nano Energy 2018, 48, 81–92. [Google Scholar] [CrossRef]
- Hu, N.; Gong, W.H.; Huang, L.; Shen, P.K. Ultrahigh energy density asymmetric electrochemical capacitors based on flower-like ZnO/Co3O4 nanobundle arrays and stereotaxically constricted graphene. J. Mater. Chem. A 2019, 7, 1273–1280. [Google Scholar] [CrossRef]
- Harilal, M.; Vidyadharan, B.; Misnon, I.I.; Anilkumar, G.M.; Lowe, A.; Ismail, J.; Yusoff, M.M.; Jose, R. One-Dimensional Assembly of Conductive and Capacitive Metal Oxide Electrodes for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 10730–10742. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Duan, S.; Fan, H.; Su, X.; Cui, Y.; Wang, R. Core@shell CoO@Co3O4 nanocrystals assembling mesoporous microspheres for high performance asymmetric supercapacitors. Chem. Eng. J. 2017, 327, 100–108. [Google Scholar] [CrossRef]
- Jinlong, L.; Meng, Y.; Tongxiang, L.; Miura, H. Facile synthesis of Co3O4@MnO2 core–shell nanocomposites for high-performance supercapacitor. Mater. Lett. 2017, 197, 127–130. [Google Scholar] [CrossRef]
- Du, G.J.; Liu, X.G.; Zong, Y.; Hor, T.S.A.; Yu, A.S.; Liu, Z.L. Co3O4 nanoparticle-modified MnO2 nanotube bifunctional oxygen cathode catalysts for rechargeable zinc-air batteries. Nanoscale 2013, 5, 4657–4661. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Wang, J.P.; Liao, F.; Han, X.R.; Xu, C.J.; Zhang, Y.F. Facile synthesis of porous Mn-doped Co3O4 oblique prisms as an electrode material with remarkable pseudocapacitance. Ceram. Int. 2019, 45, 8008–8016. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, J.; Chen, L.; Tang, S.; Deng, M.; Du, Y. All-solid-state asymmetric supercapacitors based on Fe-doped mesoporous Co3O4 and three-dimensional reduced graphene oxide electrodes with high energy and power densities. Nanoscale 2017, 9, 15423–15433. [Google Scholar] [CrossRef]
- Deng, S.; Xiao, X.; Chen, G.; Wang, L.; Wang, Y. Cd doped porous Co3O4 nanosheets as electrode material for high performance supercapacitor application. Electrochim. Acta 2016, 196, 316–327. [Google Scholar] [CrossRef]
- Li, G.M.; Chen, M.Z.; Ouyang, Y.; Yao, D.; Li, L.; Wang, L.; Xia, X.F.; Lei, W.; Chen, S.M.; Mandler, D.; et al. Manganese doped Co3O4 mesoporous nanoneedle array for long cycle-stable supercapacitors. Appl. Surf. Sci. 2019, 469, 941–950. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Reddy, M.V.; Harilal, M.; Vidyadharan, B.; Misnon, I.I.; Rahim, M.H.A.; Ismail, J.; Jose, R. Characterization of MgCo2O4 as an electrode for high performance supercapacitors. Electrochim. Acta 2015, 161, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Vadiyar, M.M.; Kolekar, S.S.; Chang, J.-Y.; Kashale, A.A.; Ghule, A.V. Reflux Condensation Mediated Deposition of Co3O4 Nanosheets and ZnFe2O4 Nanoflakes Electrodes for Flexible Asymmetric Supercapacitor. Electrochim. Acta 2016, 222, 1604–1615. [Google Scholar] [CrossRef]
- Zhao, L.G.; Yang, M.; Zhang, Z.Q.; Ji, Y.; Teng, Y.F.; Feng, Y.; Liu, X.Y. Hierarchical micro/nanostructured Co3O4@MnCo2O4 core-shell nanowire arrays on Ni foam for electrochemical energy storage. Inorg. Chem. Commun. 2018, 89, 22–26. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, X.; Xu, X.; Gao, G.; Ding, S.; Li, J.; Li, B. Preparation of scale-like nickel cobaltite nanosheets assembled on nitrogen-doped reduced graphene oxide for high-performance supercapacitors. Carbon 2014, 80, 222–228. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Yang, S.; Guo, Z.; Feng, C.; Tang, X. Synthesis and electrochemical performance of ZnCo2O4 for lithium-ion battery application. Electrochim. Acta 2015, 155, 297–304. [Google Scholar] [CrossRef]
- Sennu, P.; Aravindan, V.; Lee, Y.S. High energy asymmetric supercapacitor with 1D@2D structured NiCo2O4@Co3O4 and jackfruit derived high surface area porous carbon. J. Power Sources 2016, 306, 248–257. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, H.; Zhao, N.; Peng, H.; Ren, X.; Wang, W.; Li, H.; Chen, G.; Zhu, Y.; Jiang, X.; et al. 3D hierarchical CoWO4/Co3O4 nanowire arrays for asymmetric supercapacitors with high energy density. Chem. Eng. J. 2018, 347, 291–300. [Google Scholar] [CrossRef]
- Cui, S.X.; Li, T.T.; Guo, C.L.; Wang, L.L.; Zhang, C.C.; Yan, Z.Y.; Wei, Y.H.; Hou, L.F.; Xu, L.C.; Jia, C.K. Synthesis of Mesoporous Co3O4/NiCo2O4 Nanorods and Their Electrochemical Study. J Nanosci. Nanotechno. 2019, 19, 47–56. [Google Scholar] [CrossRef]
- Yang, F.; Xu, K.B.; Hu, J.Q. Hierarchical multicomponent electrode with NiMoO4 nanosheets coated on Co3O4 nanowire arrays for enhanced electrochemical properties. J. Alloys Compd. 2019, 781, 1127–1131. [Google Scholar] [CrossRef]
- Xu, K.; Yang, X.; Yang, J.; Hu, J. Synthesis of hierarchical Co3O4@NiCo2O4 core-shell nanosheets as electrode materials for supercapacitor application. J. Alloys Compd. 2017, 700, 247–251. [Google Scholar] [CrossRef]
- Dong, T.; Li, M.; Wang, P.; Yang, P. Synthesis of hierarchical tube-like yolk-shell Co3O4@NiMoO4 for enhanced supercapacitor performance. Int. J. Hydrogen Energy 2018, 43, 14569–14577. [Google Scholar] [CrossRef]
- Zhang, M.C.; Fan, H.Q.; Ren, X.H.; Zhao, N.; Peng, H.J.; Wang, C.; Wu, X.B.; Dong, G.Z.; Long, C.B.; Wang, W.J.; et al. Study of pseudocapacitive contribution to superior energy storage of 3D heterostructure CoWO4/Co3O4 nanocone arrays. J. Power Sources 2019, 418, 202–210. [Google Scholar] [CrossRef]
- Hu, X.W.; Liu, S.; Qu, B.T.; You, X.Z. Starfish-shaped Co3O4/ZnFe2O4 Hollow Nanocomposite: Synthesis, Supercapacity, and Magnetic Properties. ACS Appl. Mater. Interfaces 2015, 7, 9972–9981. [Google Scholar] [CrossRef] [PubMed]
- Sapna; Budhiraja, N.; Kumar, V.; Singh, S.K. Synergistic effect in structural and supercapacitor performance of well dispersed CoFe2O4/Co3O4 nano-hetrostructures. Ceram. Int. 2018, 44, 13806–13814. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, X.; Liu, Y.X.; Zhu, Y.M.; Zhuang, G.C.; Zheng, W.; Cai, Z.Y.; Yang, P.Z. Advanced binder-free electrodes based on CoMn2O4@Co3O4 core/shell nanostructures for high-performance supercapacitors. RSC Adv. 2018, 8, 31594–31602. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-J.; Han, X.; Tao, K.; Li, Q.; Li, Y.-L.; Chen, C.; Han, L. Shish-kebab type MnCo2O4@Co3O4 nanoneedle arrays derived from MnCo-LDH@ZIF-67 for high-performance supercapacitors and efficient oxygen evolution reaction. Chem. Eng. J. 2018, 354, 875–884. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, A.; Pan, T.; Dou, Y.; Shao, M.; Han, J.; Wei, M. Ultrahigh-rate-capability of a layered double hydroxide supercapacitor based on a self-generated electrolyte reservoir. J. Mater. Chem. A 2016, 4, 8421–8427. [Google Scholar] [CrossRef]
- Qorbani, M.; Naseri, N.; Moshfegh, A.Z. Hierarchical Co3O4/Co(OH)2 Nanoflakes as a Supercapacitor Electrode: Experimental and Semi-Empirical Model. ACS Appl. Mater. Interfaces 2015, 7, 11172–11179. [Google Scholar] [CrossRef]
- Pan, X.X.; Ji, F.Z.; Kuang, L.P.; Liu, F.; Zhang, Y.; Chen, X.M.; Alameh, K.; Ding, B.F. Synergetic Effect of Three-Dimensional Co3O4@Co(OH)2 Hybrid Nanostructure for Electrochemical Energy Storage. Electrochim. Acta 2016, 215, 298–304. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Jing, X.; Liu, P.; Wang, J.; Li, R. Hierarchical Co3O4@Ni(OH)2 core-shell nanosheet arrays for isolated all-solid state supercapacitor electrodes with superior electrochemical performance. Chem. Eng. J. 2017, 315, 35–45. [Google Scholar] [CrossRef]
- Su, D.; Tang, Z.; Xie, J.; Bian, Z.; Zhang, J.; Yang, D.; Zhang, D.; Wang, J.; Liu, Y.; Yuan, A.; et al. Co, Mn-LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors. Appl. Surf. Sci. 2019, 469, 487–494. [Google Scholar] [CrossRef]
- Zhou, J.J.; Li, Q.; Chen, C.; Li, Y.L.; Tao, K.; Han, L. Co3O4@CoNi-LDHcore/shell nanosheet arrays for high-performance battery-type supercapacitors. Chem. Eng. J. 2018, 350, 551–558. [Google Scholar] [CrossRef]
- Quan, W.; Xu, Y.; Wang, Y.; Meng, S.; Jiang, D.; Chen, M. Hierarchically structured Co3O4@glucose-modified LDH architectures for high-performance supercapacitors. Appl. Surf. Sci. 2019, 488, 639–647. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.C.; Qi, W.; Li, Y.T.; Wu, Y.; Zhou, S.X.; Huang, S.M.; Wei, J.; Li, H.J.; Yao, P. Binder-free Co3O4@NiCoAl-layered double hydroxide core-shell hybrid architectural nanowire arrays with enhanced electrochemical performance. Appl. Surf. Sci. 2016, 363, 381–388. [Google Scholar] [CrossRef]
- Chen, J.S.; Guan, C.; Gui, Y.; Blackwood, D.J. Rational Design of Self-Supported Ni3S2 Nanosheets Array for Advanced Asymmetric Supercapacitor with a Superior Energy Density. ACS Appl. Mater. Interfaces 2017, 9, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhang, T.; He, Y.; Jia, C.; Saha, P.; Cheng, Q. Co3O4@CoS core-shell nanosheets on carbon cloth for high performance supercapacitor electrodes. Materials 2017, 10, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Li, K.; Chen, X.; Yang, Y.; Lee, J.-M. Heterojunction-Assisted Co3S4@Co3O4 Core–Shell Octahedrons for Supercapacitors and Both Oxygen and Carbon Dioxide Reduction Reactions. Small 2017, 13, 1701724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, J.; Wu, J.; Xu, R.; Lai, M.; Gong, C.; Chen, X.; Zhou, P. Excellent Electrochemical Performance Hierarchical Co3O4@Ni3S2 core/shell nanowire arrays for Asymmetric Supercapacitors. Electrochim. Acta 2016, 207, 87–96. [Google Scholar] [CrossRef]
- Patil, D.S.; Pawar, S.A.; Shin, J.C. Core-shell structure of Co3O4@CdS for high performance electrochemical supercapacitor. Chem. Eng. J. 2018, 335, 693–702. [Google Scholar] [CrossRef]
- Pawar, S.A.; Patil, D.S.; Shin, J.C. Designing a Copper- and Silver-Sulfide Composite with Co3O4 for High-Performance Electrochemical Supercapacitors. Chemelectrochem 2019, 6, 522–534. [Google Scholar] [CrossRef]
- Feng, X.S.; Huang, Y.; Li, C.; Chen, X.F.; Zhou, S.H.; Gao, X.G.; Chen, C. Controllable synthesis of porous NiCo2O4/NiO/Co3O4 nanoflowers for asymmetric all-solid-state supercapacitors. Chem. Eng. J. 2019, 368, 51–60. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Xu, Z.; Wang, D.; Ban, C.; Zhang, H. Unique porous Mn2O3/C cube decorated by Co3O4 nanoparticle: Low-cost and high-performance electrode materials for asymmetric supercapacitors. Electrochim. Acta 2018, 289, 72–81. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.H.; Liu, C.; Luo, R.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J. Metal-organic framework derived Co3O4/C@SiO2 yolk-shell nanoreactors with enhanced catalytic performance. J. Mater. Chem. A 2018, 6, 11226–11235. [Google Scholar] [CrossRef]
- Dai, S.; Yuan, Y.; Yu, J.; Tang, J.; Zhou, J.; Tang, W. Metal-organic framework-templated synthesis of sulfur-doped core-sheath nanoarrays and nanoporous carbon for flexible all-solid-state asymmetric supercapacitors. Nanoscale 2018, 10, 15454–15461. [Google Scholar] [CrossRef] [PubMed]
- Abidin, S.; Mamat, M.S.; Rasyid, S.A.; Zainal, Z.; Sulaiman, Y. Electropolymerization of poly(3,4-ethylenedioxythiophene) onto polyvinyl alcohol-graphene quantum dot-cobalt oxide nanofiber composite for high-performance supercapacitor. Electrochim. Acta 2018, 261, 548–556. [Google Scholar] [CrossRef]
- Li, S.T.; Duan, Y.A.; Teng, Y.; Fan, N.; Huo, Y.Q. MOF-derived tremelliform Co3O4/NiO/Mn2O3 with excellent capacitive performance. Appl. Surf. Sci. 2019, 478, 247–254. [Google Scholar] [CrossRef]
- Zhou, S.S.; Hao, C.; Wang, J.J.; Wang, X.H.; Gao, H.W. Metal-organic framework templated synthesis of porous NiCo2O4/ZnCo2O4/Co3O4 hollow polyhedral nanocages and their enhanced pseudocapacitive properties. Chem. Eng. J. 2018, 351, 74–84. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Chen, K.; Cui, S.; Chen, W.; Mi, L. Synergistic effect of Co3O4@C@MnO2 nanowire heterostructures for high-performance asymmetry supercapacitor with long cycle life. Electrochim. Acta 2018, 283, 1087–1094. [Google Scholar] [CrossRef]
- Su, F.; Lyu, X.; Liu, C.; Miao, M. Flexible two-ply yarn supercapacitors based on carbon nanotube/stainless steel core spun yarns decorated with Co3O4 nanoparticles and MnOx composites. Electrochim. Acta 2016, 215, 535–542. [Google Scholar] [CrossRef]
- Jiang, L.L.; Li, Y.J.; Luo, D.; Zhang, Q.Y.; Cai, F.G.; Wan, G.J.; Xiong, L.; Ren, Z.F. Freestanding RGO-Co3O4-PPy Composite Films as Electrodes for Supercapacitors. Energy Tecnol. 2019, 7, 1800606. [Google Scholar] [CrossRef]
- Yan, S.; Xu, L.N.; Jiang, J.; Xiao, H.P.; Li, X.H. An extra-long-life supercapacitor based on Co3O4/NiCo2O4/NiO/C&S composite by decomposition of Co/Ni-based coordination complex. J. Alloys Compd. 2018, 764, 684–690. [Google Scholar]












| Synthetic Method | Material Structure | Specific Capacitance | Rate | Cycle Life Retention | Ref. |
|---|---|---|---|---|---|
| Hydrothermal | Nanosheet | 610 F·g−1 at 1 A·g−1 | 65.7% at 10 A·g−1 | 94.5% after 3000 cycles | [37] |
| Hydrothermal | Nanoflake | 1500 F·g−1 at 1 A·g−1 | 55.2% at 10 A·g−1 | 99.3% after 2000 cycles | [39] |
| Solvothermal | Nanoparticle | 523.0 F·g−1 at 0.5 A·g−1 | 66.9% at 5 A·g−1 | 104.9% after 1500 cycles. | [42] |
| Solvothermal | Nanosphere | 837.7 F·g−1 at 1 A·g−1 | 93.6% at 10 A·g−1 | 87.0% after 2000 cycles | [43] |
| Sol–gel | Nanoparticle | 120 F·g−1 at 1 A·g−1 | _ | _ | [46] |
| Sol–gel | Netlike | 708 F·g−1 at 5 mV·s−1 | 71.9% at 50 mV·s−1 | _ | [48] |
| Thermal decomposition | Nanowire | 2815.7 F·g−1 at 1 A·g−1 | 27.2% at 20 A·g−1 | 88.8% after 1100 cycles | [50] |
| Thermal decomposition | Nanoflake | 576.8 F·g−1 at 1 A·g−1 | 49.2% at 50 A·g−1 | 82% after 5000 cycles. | [51] |
| Chemical precipitation | Nanonet | 739 F·g−1 at 1 A·g−1 | 72.1% at 15 A·g−1 | 90.2% after 1000 cycles | [54] |
| Chemical precipitation | Ultralayer | 548 F·g−1 at 8 A·g−1 | 66% at 32 A·g−1 | 98.5% after 2000 cycles | [56] |
| Electrodeposition | Nanoplate | 517 F·g−1 at 1 A·g−1 | 39.1% at 20 A·g−1 | 91% after 3000 cycles. | [58] |
| Electrodeposition | Nanosheet | 6469 F·g−1 at 5 mA·cm−2 | 63.8% at 15 mA·cm−2 | 81.6% after 2000 cycles | [60] |
| Chemical bath deposition | Nanowire | 850 F·g−1 at 5 mV·s−1 | ~85 at 100 mV·s−1 | 86% after 5000 cycles. | [62] |
| Chemical bath deposition | Nanorod | 387.3 F·g−1 at 1 A·g−1 | 33.6% at 5 A·g−1 | 88% after 1000 cycles. | [63] |
| Template | Ultrathin Nanosheet | 1121 F·g−1 at 1 A·g−1 | 77.9% at 25 A·g−1 | 98.2% after 6000 cycles | [66] |
| Template | Ultrathin Nanosheet | 1216.4 F·g−1 at 1 A·g−1 | 76.1% at 20 A·g−1 | 86.4% after 8000 cycles | [67] |
| Spray pyrolysis | Thin film | 412 F·g−1 at 1 A·g−1 | 93% at 4 A·g−1 | 92.6% after 1000 cycles | [69] |
| Chemical vapor deposition | Nanosphere | 128 F·g−1 at 10 mV·s−1 | ~90% at 20 A·g−1 | >100% after 4000 cycles | [70] |
| Electrospinning technique | Nanofiber | 340 F·g−1 at 1 A·g−1 | 87.1% at 10 A·g-1 | 94% after 1000 cycles | [71] |
| Galvanic displacement | Ultrathin nanosheet | 1095 F·g−1 at 1 A·g−1 | 61.9% at 15 A·g−1 | 71% after 2000 cycles | [72] |
| Laser ablation | Nanosheet | 762 F·g−1 at 6 A·g−1 | 82.7% at 36 A·g−1 | - | [73] |
| In-site self-organization | Nanorods | 1486 F·g−1 at 1 A·g−1 | 72.9% at 15 A·g−1 | 98.8% after 5000 cycles | [74] |
| Materials | Co3O4 Structure | Specific Capacitance | Rate | Cycle life Retention | Ref. |
|---|---|---|---|---|---|
| Co3O4/SWCNT | Porous nanoflake | 313.9 F·g−1 at 1 mV·s−1 | 39.6% at 20 mV·s−1 | 80% after 3000 cycles | [78] |
| Co3O4/MWCNT | Nanofiber | 406 F·g−1 at 2 A·g−1 | 41.9% at10 A·g−1 | 93% after 10,000 cycles | [80] |
| Co3O4/CF | Nanoparticle | 586 F·g−1 at 1 A·g−1 | 66% at 50 A·g−1 | 74% after 2000 cycles | [83] |
| Co3O4/CF | Nanoparticle | 948.9 F·g−1 at 0.5 A·g−1 | 48.2% at 40 A·g−1 | 88% after 6000 cycles | [85] |
| Co3O4/AC | Nanoparticle | 491 F·g−1 at 0.1 A·g−1 | 82% at 5 A·g−1 | 89% after 5000 cycles | [91] |
| Co3O4/TC | Nanoparticle | 885 F·g−1 at 2.5 A·g−1 | 23.7% at 20 A·g−1 | 94% over 10,000 cycles | [96] |
| Co3O4/CA | Ultrafine nanoparticle | 616 F·g−1 at 1 A·g−1 | 72.2% at 20 A·g−1 | 93.6% after 5000 cycles | [100] |
| Co3O4/CA | Nanowire | 1167.6 F·g−1 at 1 A·g−1 | 42.8% at 50 A·g−1 | 92.4% after 10,000 cycles | [101] |
| Co3O4/grapheme | Nanofiber | 1935 F·g−1 at 1 A·g−1 | 72.9% at 50 A·g−1 | 83% after 2000 cycles | [34] |
| Co3O4/grapheme | Flower-like microsphere | 1625.6 F·g−1 at 0.5 A·g−1 | - | 87% after 5000 cycles | [108] |
| Co3O4/PANI | Spherical nanoparticle | 1184 F·g−1 at 1.25 A·g−1 | 42.2% at 50 A·g−1 | 84.9% after 1000 cycles | [113] |
| Co3O4/PANI | Nanocage particle | 1301 F·g−1 at 1 A·g−1 | 62.6% at 10 A·g−1 | 90% after 2000 cycles | [114] |
| Co3O4/PPy | Nanowire | 2122 F·g−1 at 5 mA·cm−2 | 53.3% at 50 mA·cm−2 | 77.8% after 5000 cycles | [118] |
| Co3O4/PPy | Nanorod | 6.67 F·cm−2 at 2 mA·cm−2 | 97.4% at 20 mA·cm−2 | ~100% after 2000 cycles | [120] |
| Co3O4/PEDOP | Nanorod | 582 F·g−1 at 0.5 A·g−1 | 69.9% at 1 A·g−1 | 78% after 5000 cycles | [122] |
| Co3O4/Pind | Nanoparticle | 1805 F·g−1 at 2 A·g−1 | 90% at 25 A·g−1 | 85% after 1000 cycles | [123] |
| Co3O4/NiO | Nanorod | 313.9 μAh·cm−2 at 4 mA·cm−2 | 76.38% at 25 mA·cm−2 | 135% after 2000 cycles | [131] |
| Co3O4/ZnO | Flower-like nanobundle | 1983 F·g−1 at 2 A·g−1 | 42% at 20 A·g−1 | 84.5% after 5000 cycles | [132] |
| Co3O4/CuO | Nanowire | 1242 F·g−1 at 2 mV·s−1 | 51% at 50 mV·s−1 | 100% after 2000 cycles | [133] |
| Co3O4/CoO | Nanomicrosphere | 3377.8 F·g−1 at 2 A·g−1 | 66.5% at 20 A·g−1 | 39.4% after 4000 cycles | [134] |
| Co3O4/MnO2 | Nanowire | 1920 F·g−1 at 1 A·g−1 | - | 95.2% after 3000 cycles | [135] |
| Mn-doping Co3O4 | Nanoneedle | 668.4 F·g−1 at 1 A·g-1 | 61.7% at 10 A·g−1 | 104% after 10,000 cycles | [155] |
| Fe-doping Co3O4 | Flowerlike nanoflake | 1997 F·g−1 at 1 A·g−1 | 61.7% at 20 A·g−1 | 92.1% after 5000 cycles | [138] |
| Co3O4/NiCo2O4 | Nanosheet | 1330 F·g−1 at 3 mA·cm−2 | 72.2% at 30 mA·cm−2 | 100.7% over 5000 cycles | [150] |
| Co3O4/NiMoO4 | Nanofiber | 998.05 F·g−1 at 0.5 A·g−1 | 88% at 20 A·g−1 | 89.9% after 3000 cycles | [151] |
| Co3O4/CoWO4 | Nanocone | 4.665 F·cm−2 at 2.7 mA·cm−2 | 70.83% at 27 mA·cm−2 | - | [152] |
| Co3O4/ZnFe2O4 | Nanocage particle | 326.7 F·g−1 at 1 A·g−1 | 51.8% at 10 A·g−1 | 80.7% after 1000 cycles | [153] |
| Co3O4/CoFe2O4 | Nanoparticle | 761.1 F·g−1 at 10 mV·s−1 | 20.9% at 50 mV·s−1 | 92.2% after 1000 cycles | [154] |
| Co3O4/CoMn2O4 | Nanosheet | 1627 F·g−1 at 1 A·g−1 | 84.6% at 10 A·g−1 | 87.6% over 3000 cycles | [155] |
| Co3O4/MnCo2O4 | Polyhedron nanoparticle | 1440 C·cm−2 at 1 mA·cm−2 | 36% at 10 mA·cm−2 | 82.76% after 8000 cycles | [156] |
| Co3O4/Co(OH)2 | Nanotube | 1876 C·g−1 at 5 mA·cm−2 | 25.4% at 25 mA·cm−2 | 83.1% over 1000 cycles | [159] |
| Co3O4/Ni(OH)2 | Nanosheet | 1306.3 F·g−1 at 1.2 A·g−1 | 46% at 12 A·g−1 | 90% after 3000 cycles | [160] |
| Co3O4/CoNi-LDH | Nanoplate | 2676.9 F·g−1 at 0.5 A·g−1 | 43% at 20 A·g−1 | 67.7% after 10,000 cycles | [162] |
| Co3O4/NiMn-LDH | Nanowire | 1644 F·g−1 at 1A·g−1 | 42.4% at 10A·g−1 | 94.2% after 5000 cycles | [163] |
| Co3O4/CoS | Nanosheet | 764.2 F·g−1 at 1.0 A·g−1 | 72.2% at 10 A·g−1 | 78.1% after 5000 cycles | [166] |
| Co3O4/Ni3S2 | Nanowire | 1710 F·g−1 at 1A·g−1 | 86.2% at 10 A·g−1 | 85.3% after 1000 cycles | [168] |
| Co3O4/CdS | Nanosheet | 1539 F·g−1 at 10mV·s−1 | 52% at 100 mV·s−1 | 98.5% after 2000 cycles | [169] |
| Co3O4/Cu2S | Nanosheet | 5324 mF·cm−2 at 10 mV·s−1 | 30.6% at 100 mV·s−1 | 98.2% after 2000 cycles | [170] |
| Co3O4/Ag2S | Nanosheet | 2243 mF·cm−2 at 10 mV·s−1 | 43.1% at 100 mV·s−1 | 96.7% after 2000 cycles | [170] |
| Co3O4/NiO/Mn2O3 | Nanosheet | 3652 mF·cm−2 at 1 mA·cm−2 | 70% at 20 mA·cm−2 | 87.6% after 10,000 cycles | [176] |
| Co3O4/NiCo2O4/ZnCo2O4 | Nanocage particle | 1892.5 F·g−1 at 1 A·g−1 | 60% at 10 A·g−1 | 66% after 2000 cycles | [177] |
| Co3O4/C/MnO2 | Lily-like nanostructures | 1561.3 F·g−1 at 0.5 A·g-1 | 85.5% at 20 A·g−1 | 95% after 10,000 cycles | [178] |
| Co3O4/CNT/SS | Nanoparticle | 82.94 F·cm−3 at 0.02 V·s−1 | 58.96 at 0.05 V·s−1 | 80.4 after 1000 cycles | [179] |
| Co3O4-PPy-rGO | Nanoparticle | 532.8 F·g−1 at 5 mV·s−1 | - | 100% after 700 cycles | [180] |
| Co3O4/NiCo2O4/NiO/C&S | Nanoparticle | 428.24 F·g−1 at 0.5 A·g−1 | 61.5% at 10 A·g−1 | 94.2% after 20,000 cycles | [181] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, A.; Meng, C.; Wu, C.; Yang, S.; Hong, X. Recent Advance in Co3O4 and Co3O4-Containing Electrode Materials for High-Performance Supercapacitors. Molecules 2020, 25, 269. https://doi.org/10.3390/molecules25020269
Wang X, Hu A, Meng C, Wu C, Yang S, Hong X. Recent Advance in Co3O4 and Co3O4-Containing Electrode Materials for High-Performance Supercapacitors. Molecules. 2020; 25(2):269. https://doi.org/10.3390/molecules25020269
Chicago/Turabian StyleWang, Xuelei, Anyu Hu, Chao Meng, Chun Wu, Shaobin Yang, and Xiaodong Hong. 2020. "Recent Advance in Co3O4 and Co3O4-Containing Electrode Materials for High-Performance Supercapacitors" Molecules 25, no. 2: 269. https://doi.org/10.3390/molecules25020269