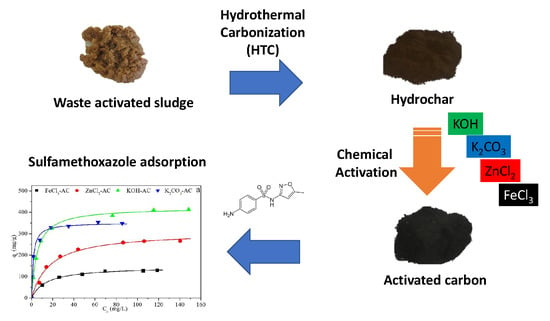
Potential Use of Waste Activated Sludge Hydrothermally Treated as a Renewable Fuel or Activated Carbon Precursor
Abstract
:1. Introduction
2. Methods
2.1. Dewatered Waste Activated Sludge
2.2. HTC and Hydrochar Activation Procedures
2.3. Characterization of Hydrochars and Activated Carbons
2.4. Adsorption Tests
3. Results and Discussion
3.1. Chemical and Structural Properties of the Hydrochars
3.2. Air Activation of Hydrochars
3.3. Chemical Activation of Hydrochars
3.4. Surface Chemistry of the Hydrochars
3.5. Adsorption of Sulfamethoxazole, Antipyrine and Desipramine.
4. Conclusions and Future Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- OECD. Information Environment Database Sewage Sludge Production and Disposal. Available online: http://www.oecd.org/ (accessed on 1 May 2020).
- Li, R.; Zhang, Z.; Li, Y.; Teng, W.; Wang, W.; Yang, T. Transformation of apatite phosphorus and non-apatite inorganic phosphorus during incineration of sewage sludge. Chemosphere 2015, 141, 57–61. [Google Scholar] [CrossRef] [PubMed]
- De Andrés, J.M.; Roche, E.; Narros, A.; Rodríguez, M.E. Characterisation of tar from sewage sludge gasification. Influence of gasifying conditions: Temperature, throughput, steam and use of primary catalysts. Fuel 2016, 180, 116–126. [Google Scholar] [CrossRef]
- Lumley, N.P.G.; Ramey, D.F.; Prieto, A.L.; Braun, R.J.; Cath, T.Y.; Porter, J.M. Techno-economic analysis of wastewater sludge gasification: A decentralized urban perspective. Bioresour. Technol. 2014, 161, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Preparation of adsorbents from sewage sludge pyrolytic char by carbon dioxide activation. Process. Saf. Environ. Prot. 2016, 103, 76–86. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Mohedano, A.F.; Rodriguez, J.J. Activated carbons from sewage sludge. Desalination 2011, 277, 377–382. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Díaz, R.M.; Xiberta, J.; Prieto, I. Volatilisation of trace elements for coal-sewage sludge blends during their combustion. Fuel 2003, 82, 1939–1948. [Google Scholar] [CrossRef]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2014, 257, 154–159. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Fornes, F.; Belda, R.M.; Fernández de Córdova, P.; Cebolla-Cornejo, J. Assessment of biochar and hydrochar as minor to major constituents of growing media for containerized tomato production. J. Sci. Food Agric. 2017, 97, 3675–3684. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal carbonization: Modeling, final properties design and applications: A review. Energies 2018, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Rubia, M.A.; Villamil, J.A.; Rodríguez, J.J.; Mohedano, A.F. Effect of inoculum source and initial concentration on the anaerobic digestion of the liquid fraction from hydrothermal carbonisation of sewage sludge. Renew. Energy 2018, 127, 697–704. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; de la Rubia, M.A. Valorisation of the liquid fraction from hydrothermal carbonisation of sewage sludge by anaerobic digestion. J. Chem. Technol. Biotechnol. 2018, 93, 450–456. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Ranzi, E.M.; Filho, R.M.; Volpe, M.; Fiori, L.; Merzari, F.; Messineo, A.; Andreottola, G. Hydrothermal Carbonization as an Efficient Tool for Sewage Sludge Valorization and Phosphorous Recovery. Chem. Eng. Trans. 2020, 80, 2020. [Google Scholar]
- Stutzenstein, P.; Weiner, B.; Köhler, R.; Pfeifer, C.; Kopinke, F.-D. Wet oxidation of process water from hydrothermal carbonization of biomass with nitrate as oxidant. Chem. Eng. J. 2018, 339, 1–6. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; Borja, R.; de la Rubia, M.A. Anaerobic co-digestion of the organic fraction of municipal solid waste and the liquid fraction from the hydrothermal carbonization of industrial sewage sludge under thermophilic conditions. Front. Sustain. Food Syst. 2018, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- De la Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Borja, R.; Mohedano, A.F. Mesophilic anaerobic co-digestion of the organic fraction of municipal solid waste with the liquid fraction from hydrothermal carbonization of sewage sludge. Waste Manag. 2018, 76, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B.; Mumme, J. Anaerobic digestion of waste water from hydrothermal carbonization of corn silage. Appl. Bioenergy 2013, 1, 1–10. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.A.; Rodriguez, J.J.; Mohedano, A.F.; de la Rubia, M.A. Valorization of microalgal biomass by hydrothermal carbonization and anaerobic digestion. Bioresour. Technol. 2018, 274, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Merzari, F.; Langone, M.; Andreottola, G.; Fiori, L. Methane production from process water of sewage sludge hydrothermal carbonization. A review. Valorising sludge through hydrothermal carbonization. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1–42. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.; Qaramaleki, S.V.; Coronella, C.J.; Mohedano, A.F.; de la Rubia, M.A. Energy valorization of cow manure by hydrothermal carbonization and anaerobic digestion. Renew. Energy 2020. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Villamil, J.A.; de la Rubia, M.A.; Diaz, E.; Mohedano, A.F. Technologies for wastewater sludge utilization and energy production: Hydrothermal carbonization of lignocellulosic biomass and sewage sludge. In Wastewater Treatment Residues as Resources for Biorefin. Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–153. [Google Scholar]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Song, E.; Park, S.; Kim, H. Upgrading Hydrothermal Carbonization (HTC) Hydrochar from Sewage Sludge. Energies 2019, 12, 2383. [Google Scholar] [CrossRef] [Green Version]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: A feasibility study on a laboratory scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Aragon-Briceno, C.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal carbonization of sewage digestate at wastewater treatment works: Influence of solid loading on characteristics of hydrochar, process water and plant energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Merzari, F.; Goldfarb, J.; Andreottola, G.; Mimmo, T.; Volpe, M.; Fiori, L. Hydrothermal carbonization as a strategy for sewage sludge management: Influence of process withdrawal point on hydrochar properties. Energies 2020, 13, 2890. [Google Scholar] [CrossRef]
- Hansen, L.J.; Fendt, S.; Spliethoff, H. Impact of hydrothermal carbonization on combustion properties of residual biomass. Biomass Convers. Biorefinery 2020, 1–12. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G.; Zhu, Y. Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Koottatep, T.; Fakkaew, K.; Tajai, N.; Pradeep, S.V.; Polprasert, C. Sludge stabilization and energy recovery by hydrothermal carbonization process. Renew. Energy 2016, 99, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, S.; Saha, P.; Reza, M.T. Co-hydrothermal carbonization of coal waste and food waste: Fuel characteristics. Biomass Convers. Biorefin. 2020, 1–11. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2018, 92, 1779–1799. [Google Scholar] [CrossRef]
- Saetea, P.; Tippayawong, N. Characterization of adsorbent from hydrothermally carbonized and steam activated sewage sludge. In Proceedings of the World Congress on Engineering; Newswood Limited: London, UK, 2013; Volume 3. [Google Scholar]
- Spataru, A.; Jain, R.; Chung, J.W.; Gerner, G.; Krebs, R.; Lens, P.N.L. Enhanced adsorption of orthophosphate and copper onto hydrochar derived from sewage sludge by KOH activation. RSC Adv. 2016, 6, 101827–101834. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Fernández Mohedano, A.; Rodríguez, J.J. Activated carbons from sewage sludge. Application to aqueous-phase adsorption of 4-chlorophenol. Desalination 2011, 277, 377–382. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rey, A.; Zazo, J.A.; Casas, J.A.; Bahamonde, A.; Rodriguez, J.J. Influence of the structural and surface characteristics of activated carbon on the catalytic decomposition of hydrogen peroxide. Appl. Catal. A Gen. 2011, 402, 146–155. [Google Scholar] [CrossRef]
- Çalışkan, E.; Göktürk, S. Adsorption characteristics of sulfamethoxazole and metronidazole on activated carbon. Sep. Sci. Technol. 2010, 45, 244–255. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, S.; Choi, J.-W.; Bediako, J.K.; Song, M.-H.; Kim, J.-A.; Cho, C.-W.; Yun, Y.-S. Prediction of adsorption properties for ionic and neutral pharmaceuticals and pharmaceutical intermediates on activated charcoal from aqueous solution via LFER model. Chem. Eng. J. 2019, 362, 199–206. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; San Martín, I.; Carrillo, P. Effect of sodium persulfate as electron acceptor on antipyrine degradation by solar TiO2 or TiO2/rGO photocatalysis. Chem. Eng. J. 2019, 364, 257–268. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- ISO. Solid Biofuels—Fuel Specifications and Classes—Part. 8: Graded Thermally Treated and Densified Biomass Fuels (17225-8:2016); ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Burguete, P.; Corma, A.; Hitzl, M.; Modrego, R.; Ponce, E.; Renz, M. Fuel and chemicals from wet lignocellulosic biomass waste streams by hydrothermal carbonization. Green Chem. 2016, 18, 1051–1060. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Repo, E.; Mäkilä, E.; Salonen, J.; Vakkilainen, E.; Sillanpää, M. Adsorption behavior of hydrothermally treated municipal sludge & pulp and paper industry sludge. Bioresour. Technol. 2013, 147, 71–76. [Google Scholar]
- Zhang, J.; Lin, Q.; Zhao, X. The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. J. Integr. Agric. 2014, 13, 471–482. [Google Scholar] [CrossRef]
- Titirici, M.M. Hydrothermal Carbonisation: A Sustainable Alternative to Versatile Carbon Materials. Ph.D. Thesis, University of Potsdam, Potsdam, Germany, 2012. [Google Scholar]
- EBC European Biochar Certificate—Guidelines for a Sustainable Production of Biochar. Available online: http://www.european-biochar.org/biochar/media/doc/ebc-guidelines.pdf (accessed on 15 May 2020).
- De Mena Pardo, B.; Doyle, L.; Renz, M.; Salimbeni, A. Industrial Scale Hydrothermal Carbonization: New Applications for Wet Biomass Waste; Ttz Bremerhaven: Bremerhaven, Germany, 2016. [Google Scholar]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards biochar and hydrochar engineering—Influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef] [Green Version]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J.; Sánchez-González, C.; Rufián-Henares, J.Á. Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.-G.; Titirici, M.-M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Müller, C.; Grünhage, L.; Koch, C.; Kammann, C. Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—Effects on greenhouse gas emissions and plant growth. Agric. Ecosyst. Environ. 2014, 191, 39–52. [Google Scholar] [CrossRef]
- Kalderis, D.; Papameletiou, G.; Kayan, B. Assessment of orange peel hydrochar as a soil amendment: Impact on clay soil physical properties and potential phytotoxicity. Waste Biomass Valoriz. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef]
- Hitzl, M.; Mendez, A.; Owsianiak, M.; Renz, M. Making hydrochar suitable for agricultural soil: A thermal treatment to remove organic phytotoxic compounds. J. Environ. Chem. Eng. 2018, 6, 7029–7034. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, L.; Li, S.; Geng, J.; Song, Q.; Liu, J.; Wang, C.; Wang, H.; Li, J.; Qin, Z.; et al. Simple approach to carboxyl-rich materials through low-temperature heat treatment of hydrothermal carbon in air. Appl. Surf. Sci. 2011, 257, 8686–8691. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One step forward toward characterization: Some important material properties to distinguish biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Benstoem, F.; Becker, G.; Firk, J.; Kaless, M.; Wuest, D.; Pinnekamp, J.; Kruse, A. Elimination of micropollutants by activated carbon produced from fibers taken from wastewater screenings using hydrothermal carbonization. J. Environ. Manag. 2018, 211, 278–286. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhao, J.; Yang, Y.; Wang, J.Y. Multiscale characteristics dynamics of hydrochar from hydrothermal conversion of sewage sludge under sub- and near-critical water. Bioresour. Technol. 2016, 211, 486–493. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Guo, Y.; Rockstraw, D.A. Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour. Technol. 2007, 98, 1513–1521. [Google Scholar] [CrossRef]
- Huang, C.-C.; Li, H.-S.; Chen, C.-H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef]
- Tamon, H.; Okazaki, M. Influence of acidic surface oxides of activated carbon on gas adsorption characteristics. Carbon NY 1996, 34, 741–746. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 111, 3973. [Google Scholar] [CrossRef]






| C (%) | 41.5 (0.1) | Na (mg/g) | 11.6 (0.2) |
| H (%) | 6.0 (0.1) | Mg (mg/g) | 0.7 (0.1) |
| N (%) | 6.8 (0.1) | Al (mg/g) | 15.7 (0.2) |
| S (%) | 0.7 (0.1) | P (mg/g) | 20.8 (0.4) |
| O (%) a | 31.3 (0.1) | K (mg/g) | 7.4 (0.1) |
| Ash content (%) | 13.7 (0.1) | Ca (mg/g) | 2.7 (0.2) |
| Volatile matter (%) | 73.6 (0.1) | Ti (mg/g) | 0.6 (0.1) |
| Fixed carbon (%) | 12.7 (0.1) | Fe (mg/g) | 0.2 (0.1) |
| Experimental Conditions | Hydrochar Yield (%) | Fixed Carbon (%) | Ash (%) | Volatile Matter (%) | C (%) | H (%) | S (%) | N (%) | O (%) a | C Recovery (%) | SBET (m2/g) | Vmesob (cm3/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 140 °C—2.3 h | 59.7 | 12.0 (0.1) | 15.8 (0.1) | 72.5 (0.3) | 39.6 (0.3) | 6.1 (0.1) | 0.3 (0.1) | 5.6 (0.1) | 32.9 (0.2) | 57.0 | <3 | 0.001 |
| 152 °C—1 h | 61.5 | 11.5 (0.1) | 15.0 (0.1) | 73.5 (0.1) | 40.6 (0.1) | 6.3 (0.1) | 0.4 (0.1) | 5.9 (0.1) | 31.7 (0.1) | 60.2 | <3 | 0.001 |
| 152 °C—3.5 h | 58.5 | 11.1 (0.1) | 17.1 (0.1) | 71.8 (3.3) | 40.4 (0.9) | 6.0 (0.1) | 0.2 (0.1) | 5.2 (0.1) | 31.3 (0.1) | 56.9 | 5 | 0.006 |
| 180 °C—0.5 h | 48.5 | 8.1 (0.1) | 16.4 (0.1) | 75.4 (1.7) | 40.5 (0.1) | 6.2 (0.1) | 0.3 (0.1) | 5.6 (0.1) | 31.1 (0.2) | 47.3 | <3 | 0.001 |
| 180 °C—2.3 h | 49.0 | 13.6 (0.4) | 19.3 (0.5) | 67.2 (0.5) | 40.7 (0.8) | 5.8 (0.1) | 0.2 (0.1) | 4.6 (0.1) | 29.5 (0.2) | 48.1 | 15 | 0.020 |
| 180 °C—4 h | 46.2 | 15.5 (0.2) | 18.7 (0.1) | 65.8 (0.1) | 42.7 (0.1) | 5.6 (01) | 0.2 (0.1) | 5.0 (0.1) | 27.7 (0.2) | 47.5 | 20 | 0.027 |
| 208 °C—1 h | 40.3 | 14.9 (0.1) | 19.7 (0.1) | 65.4 (0.3) | 43.1 (0.2) | 5.8 (0.1) | 0.2 (0.1) | 4.6 (0.1) | 26.5 (0.3) | 41.9 | 21 | 0.026 |
| 208 °C—3.5 h | 37.7 | 15.4 (0.1) | 21.3 (0.1) | 63.2 (0.1) | 43.6 (0.1) | 5.5 (0.1) | 0.3 (0.1) | 4.5 (0.1) | 24.9 (0.1) | 39.6 | 23 | 0.032 |
| 220 °C—2.3 h | 31.6 | 15.8 (0.2) | 22.8 (0.1) | 63.3 (1.6) | 41.5 (0.1) | 5.3 (0.1) | 0.2 (0.1) | 4.1 (0.1) | 26.1 (0.2) | 31.6 | 24 | 0.031 |
| Equation | R2 (%) | F-Value | Number |
|---|---|---|---|
| HHV (MJ/kg) = 13.256 + 0.03662·T + 0.361·t | 0.882 | 37.2 | (5) |
| Ash content (wt.%) = 0.09392·T + 0.782·t | 0.998 | 3386.2 | (6) |
| Hydrochar yield (wt.%) = 0.9157·T − 0.00354·T2 | 0.997 | 2246.2 | (7) |
| C content (wt.%) = 0.4217·T − 0.00106·T2 | 0.999 | 7420.8 | (8) |
| N content (wt.%) = 0.07253·T − 0.00025·T2 | 0.994 | 929.4 | (9) |
| T temperature (°C). t reaction time (h) | |||
| Preparation Conditions | HHV (MJ/kg) | Energy Density | Energy Yield (%) |
|---|---|---|---|
| 140 °C—2.3 h | 19.3 (0.1) | 1.10 | 65.5 |
| 152 °C—1 h | 19.1 (0.1) | 1.09 | 66.7 |
| 152 °C—3.5 h | 19.9 (0.1) | 1.13 | 66.1 |
| 180 °C—0.5 h | 19.5 (0.1) | 1.11 | 53.7 |
| 180 °C—2.3 h | 20.8 (0.2) | 1.18 | 57.9 |
| 180 °C—4 h | 21.6 (0.1) | 1.23 | 56.7 |
| 208 °C—1 h | 21.6 (0.1) | 1.23 | 49.5 |
| 208 °C—3.5 h | 21.4 (0.5) | 1.22 | 45.8 |
| 220 °C—2.3 h | 22.3 (0.1) | 1.27 | 40.0 |
| Material | Slurry pH | T (°C) | Ash (%) | Fixed Carbon (%) | Elemental Composition (%) a | SBET (m2/g) | Vmicrob (cm3/g) | Vmesoc (cm3/g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | S | ||||||||
| K2CO3-AC | 5.5 | 650 | 13.1 (0.5) | 55.0 (1.3) | 61.1 (0.6) | 5.8 (0.3) | 0.2 (0.1) | 583 | 0.235 | 0.189 |
| 850 | 43.4 (0.8) | 28.9 (1.0) | 34.9 (1.0) | 0.5 (0.1) | 0.1 (0.1) | 832 | 0.290 | 0.268 | ||
| KOH-AC | 6.6 | 650 | 18.0 (0.5) | 52.0 (1.4) | 60.9 (2.7) | 7.4 (0.2) | 0.7 (0.1) | 402 | 0.162 | 0.079 |
| 850 | 10.9 (0.4) | 67.2 (1.7) | 81.0 (3.8) | 1.3 (0.1) | 0.1 (0.1) | 968 | 0.354 | 0.271 | ||
| FeCl3-AC | 5.1 | 650 | 37.6 (0.7) | 23.8 (0.5) | 39.9 (0.1) | 4.8 (0.2) | 0.1 (0.1) | 443 | 0.179 | 0.098 |
| 850 | 58.4 (1.2) | 7.5 (0.3) | 28.9 (0.2) | 1.8 (0.1) | 0.2 (0.1) | 411 | 0.136 | 0.146 | ||
| ZnCl2-AC | 5.7 | 650 | 9.4 (0.3) | 65.9 (1.3) | 66.4 (0.1) | 6.9 (0.2) | 0.3 (0.1) | 661 | 0.249 | 0.145 |
| 850 | 18.6 (0.6) | 50.1 (1.6) | 57.6 (3.0) | 5.3 (0.2) | 0.6 (0.1) | 1030 | 0.398 | 0.204 | ||
| Sulfamethoxazole | ||||
| Parameter | FeCl3-AC | ZnCl2-AC | KOH-AC | K2CO3-AC |
| qL (mg/g) | 145.8 (2.3) | 309.2 (9.6) | 422.9 (3.9) | 350.5 (7.1) |
| KL (L/mg) | 0.07 (0.01) | 0.06 (0.01) | 0.19 (0.01) | 0.68 (0.09) |
| R2 | 0.997 | 0.991 | 0.998 | 0.991 |
| Antipyrine | ||||
| Parameter | FeCl3-AC | ZnCl2-AC | KOH-AC | K2CO3-AC |
| qL (mg/g) | 50.0 (2.4) | 64.5 (2.6) | 212.6 (2.2) | 200.7 (8.7) |
| KL (L/mg) | 0.01 (<0.01) | 0.02 (<0.01) | 0.05 (<0.01) | 0.01 (<0.01) |
| R2 | 0.992 | 0.986 | 0.998 | 0.989 |
| Desipramine | ||||
| Parameter | FeCl3-AC | ZnCl2-AC | KOH-AC | K2CO3-AC |
| qL (mg/g) | 65.9 (3.1) | 95.8 (2.0) | 160.7 (8.5) | 132.1 (4.4) |
| KL (L/mg) | 0.29 (0.06) | 1.00 (0.10) | 0.40 (0.09) | 0.67 (0.11) |
| R2 | 0.982 | 0.995 | 0.971 | 0.983 |
| Errors represent the 95% confidence interval. | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamil, J.A.; Diaz, E.; de la Rubia, M.A.; F. Mohedano, A. Potential Use of Waste Activated Sludge Hydrothermally Treated as a Renewable Fuel or Activated Carbon Precursor. Molecules 2020, 25, 3534. https://doi.org/10.3390/molecules25153534
Villamil JA, Diaz E, de la Rubia MA, F. Mohedano A. Potential Use of Waste Activated Sludge Hydrothermally Treated as a Renewable Fuel or Activated Carbon Precursor. Molecules. 2020; 25(15):3534. https://doi.org/10.3390/molecules25153534
Chicago/Turabian StyleVillamil, J. A., E. Diaz, M. A. de la Rubia, and A. F. Mohedano. 2020. "Potential Use of Waste Activated Sludge Hydrothermally Treated as a Renewable Fuel or Activated Carbon Precursor" Molecules 25, no. 15: 3534. https://doi.org/10.3390/molecules25153534