Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy
Abstract
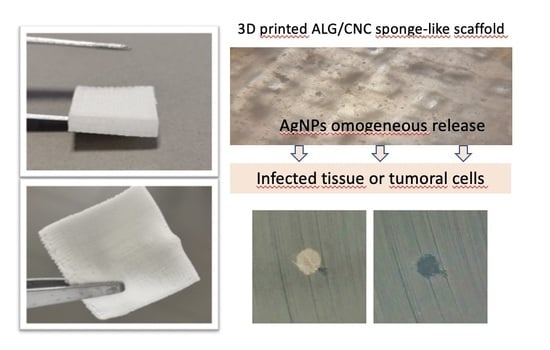
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ink Preparation for 3D Printing
2.2.2. Three-Dimensional (3D) Printing and Scaffold Production
2.2.3. Scaffold Characterization
2.2.4. Elemental (Ag) Distribution within Scaffolds and Overall Morphology
2.2.5. Antimicrobial Activity
2.2.6. Cytotoxic Activity towards Hepatocellular Carcinoma (HepG2) Cells
HepG2 Seeding on Scaffolds
Viability MTT Assay
2.2.7. Cells Collection for Proteomic Analysis
2.2.8. Protein Extraction and Sample Preparation
2.2.9. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.2.10. Software for Bioinformatic Analysis and Mass Spectrometry (MS) Data Processing
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Three-Dimensional (3D) Scaffold Preparation and Characterization
3.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
3.3. Antimicrobial Activity Assay
3.4. Cytotoxic Activity Towards HepG2 Cells
3.4.1. Cell Viability Evaluation
3.4.2. Evaluation of Apoptosis Induction through LC-MS/MS Protein Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Scott Van Epps, J.; Younger, J.G. Implantable Device Related Infection. Shock 2016, 46, 597–608. [Google Scholar]
- Misic, A.M.; Gardner, S.E.; Grice, E.A. The wound microbiome: Modern approaches to examining the role of microorganisms in impaired chronic wound healing. Adv. Wound Care 2014, 3, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. J. Expert Rev. Anti-Infect. Ther. 2013, 3, 605–613. [Google Scholar]
- Hartemann-Heurtiera, A.; Senneville, E. Diabetic foot osteomyelitis. Diabetes Metab. 2008, 34, 87–95. [Google Scholar] [CrossRef]
- Franks, L.A.; Slansky, J.E. Multiple Associations between A Broad Spectrum of Autoimmune Diseases, Chronic Inflammatory Diseases and Cancer. Anticancer Res. 2012, 32, 1119–1136. [Google Scholar]
- Percival, S.L.; Bowler, P.G.; Dolman, J. Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int. Wound J. 2007, 4, 186–191. [Google Scholar] [CrossRef]
- Jones, A.; Vaughan, D. Hydrogel dressings in the management of a variety of wound types: A review. J. Orthop. Nurs. 2005, 9, S1–S11. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [Green Version]
- Hunta, N.C.; Smith, A.M.; Gbureck, U.; Shelton, R.M.; Grover, L.M. Encapsulation of fibroblasts causes accelerated alginate hydrogel degradation. Acta Biomater. 2010, 6, 2649–3656. [Google Scholar] [CrossRef]
- Dhivya, S.; Vijaya Padma, V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22–28. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knill, C.J.; Kennedy, J.F.; Mistry, J.; Miraftab, M.; Smart, G.; Groocock, M.R.; Williams, H.J. Alginate fibres modified with unhydrolysed and hydrolysed chitosans for wound dressings. Carbohydr. Polym. 2004, 55, 65–76. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, R.; Zhao, X.; Ji, Q.; Xing, Y.; Sunarso, J.; Xia, Y. Biopolymer composite fibres composed of calcium alginate reinforced with nanocrystalline cellulose. Compos. Part A Appl. Sci. Manuf. 2017, 96, 155–163. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Ahmed, A.; Boateng, J. Calcium alginate-based antimicrobial film dressings for potential healing of infected foot ulcers. Ther. Deliv. 2018, 9, 185–204. [Google Scholar] [CrossRef]
- Inbathamizh, L.; Ponnu, T.M.; Mary, E.J. In vitro evaluation of antioxidant and anticancer potential of Morinda pubescens synthesized silver nanoparticles. J. Pharm. Res. 2013, 6, 32–38. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dar, M.Y.; Joshi, B.; Sharma, B.; Shrivastava, S.; Shukla, S. Phytofabrication of Silver nanoparticles: Novel Drug to overcome hepatocellular ailments. Toxicol. Rep. 2018, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef]
- Tiwari, A.; Tiwari, A. Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering; John Wiley Sons: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, T.S.; Wong, C.H.; Hong, C.L.; Tsai, Y.H.; Liang, C.C.; Lu, F.J.; Chang, W.H. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem. Toxicol. 2009, 47, 638–644. [Google Scholar] [CrossRef]
- Wolf-Yadlin, A.; Hautaniemi, S.; Lauffenburger, D.A.; White, F.M. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. PNAS 2007, 104, 5860–5865. [Google Scholar] [CrossRef] [Green Version]
- Bergamonti, L.; Potenza, M.; Poshtiri, A.H.; Lorenzi, A.; Sanangelantoni, A.M.; Lazzarini, L.; Lottici, P.P.; Graiff, C. Ag-functionalized nanocrystalline cellulose for paper preservation and strengthening. Carbohydr. Polym. 2020, 231, 115773. [Google Scholar] [CrossRef]
- Basile, R.; Bergamonti, L.; Fernandez, F.; Graiff, C.; Haghighi, A.; Isca, C.; Lottici, P.P.; Pizzo, B.; Predieri, G. Bio-inspired consolidants derived from crystalline nanocellulose for decayed wood. Carbohydr. Polym. 2018, 202, 164–171. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Bergonzi, C.; Di Natale, A.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R.; Elviri, L. Study of 3D-printed chitosan scaffold features after different post-printing gelation processes. Sci. Rep. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W. Molecular basis of calcium binding by polyguluronate chains. Revising the egg-box model. J. Comput. Chem. 2011, 32, 2988–2995. [Google Scholar] [CrossRef]
- Godebo, G.; Kibru, G.; Tassew, H. Multidrug-resistant bacterial isolates in infected wounds at Jimma University Specialized Hospital, Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biemer, J.J. Antimicrobial Susceptibility Testing by the Kirby-Bauer Disc Diffusion Method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar] [PubMed]
- Stanley, B.A.; Neverova, I.; Brown, H.A.; Van Eyk, J.E. Optimizing protein solubility for two-dimensional gel-electrophoresis analysis of human myocardium. Proteomics 2003, 3, 815–820. [Google Scholar] [CrossRef]
- Qin, Y. Absorption characteristics of alginate wound dressings. J. Appl. Polym. Sci. 2004, 91, 953–957. [Google Scholar] [CrossRef]
- Akhtar, R.; Sherratt, M.J.; Kennedy Cruickshank, J.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105. [Google Scholar] [CrossRef]








| SCAFFOLD | Staphylococcus aureus | Pseudomonas aeruginosa | ||
|---|---|---|---|---|
| Ø Inhibition Diameter (mm) | ||||
| ALG | 0 | 0 | 0 | 0 |
| ALG/CNC | 0 | 0 | 0 | 0 |
| ALG/CNC + AgNP (100 μg/mL) | 6 | 6 | 6 | 6 |
| ALG/CNC + AgNP (10 μg/mL) | 6 | 6 | 6 | 6 |
| ALG/CNC + AgNP (5 μg/mL) | 0 | 0 | 0 | 0 |
| ALG/CNC + AgNP (1 μg/mL) | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials 2020, 10, 844. https://doi.org/10.3390/nano10050844
Bergonzi C, Remaggi G, Graiff C, Bergamonti L, Potenza M, Ossiprandi MC, Zanotti I, Bernini F, Bettini R, Elviri L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials. 2020; 10(5):844. https://doi.org/10.3390/nano10050844
Chicago/Turabian StyleBergonzi, Carlo, Giulia Remaggi, Claudia Graiff, Laura Bergamonti, Marianna Potenza, Maria Cristina Ossiprandi, Ilaria Zanotti, Franco Bernini, Ruggero Bettini, and Lisa Elviri. 2020. "Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy" Nanomaterials 10, no. 5: 844. https://doi.org/10.3390/nano10050844